BRACO 19 synthesis
- Product Name:BRACO 19
- CAS Number:351351-75-2
- Molecular formula:C35H43N7O2
- Molecular Weight:593.76
Yield:351351-75-2 88%
Reaction Conditions:
in chloroform; for 2 h;
Steps:
55
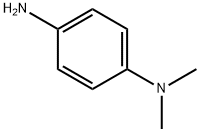
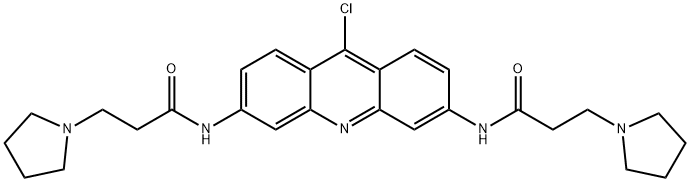
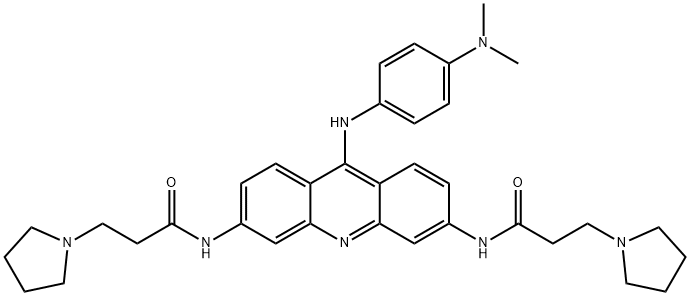
9Substitution General Procedure [0633] To a vigorously stirred solution of BR-ACO-18 in CHCl3 was added dropwise a solution of the proposed amine, Q-NH2 (e.g., NH2(CH2)pNH2, substituted anilines), for example7 in CHCl3. This solution was stirred for a further 2 hr, the solvent was then removed under reduced pressure and the resultant solid washed with EtOH and Et2O to give the desired trisubstituted derivative. Example 55 N-{9-[4'-(N,N-Dimethylamino)phenylamino]}-3,6-bis(3-pyrrolidinopropionamido)acridine (BR-ACO-21) [0636] 3,6-Bisamido-9-chloroacridone BR-ACO-18 (400 mg, 0.8 mmol) was treated with 4-N,N-dimethylaminoaniline (0.5 mL) according to the general procedure to give the desired product BR-ACO-21 (400 mg, 88%) as a brown solid. [0637] Mp >320° C., 1H-NMR (DMSO) δ1.81 (8 H, m, N(CH2CH2)2), 2.81 (4H, t, J 6.9 Hz. COCH2CH2N), 2.85 (8H, m, N(CH2CH2)2), 3.16 (4H, t, J 6.9 Hz, COCH2CH2N), 6.67 (2H, d, J 8.7 Hz, H-2,7), 6.96 (2H, d, J 8.7 Hz, H-1,8), 7.26 (2H, d, J 8.6 Hz, H-3',5'), 8.03 (2H, d, J 8.6 Hz, H-2',6'), 8.34 (2H, s, H-4,5), 10.92 (2H, s, NHCO), m/z (EI) 594.3540 (requires C35H40N7O2 M+H 594.3556).
References:
US2003/207909,2003,A1 Location in patent:Page/Page column 48
351351-78-5
0 suppliers
inquiry

351351-75-2
8 suppliers
inquiry

1817-76-1
13 suppliers
inquiry

351351-75-2
8 suppliers
inquiry
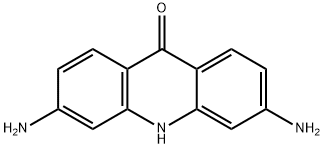
42832-87-1
13 suppliers
$72.00/50mg

351351-75-2
8 suppliers
inquiry

71535-97-2
10 suppliers
inquiry

351351-75-2
8 suppliers
inquiry