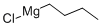Brivaracetam Impurity 30 synthesis
- Product Name:Brivaracetam Impurity 30
- CAS Number:22530-99-0
- Molecular formula:C8H14O2
- Molecular Weight:142.2
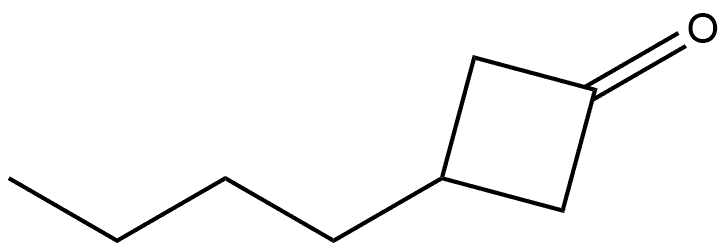
69906-54-3
0 suppliers
inquiry

22530-99-0
9 suppliers
inquiry
Yield:22530-99-0 93%
Reaction Conditions:
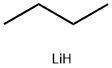
with bis-trimethylsilanyl peroxide;1-hexyl-3-methylimidazolium chloroaluminate(III) at 0 - 20; for 3 h;Acidic conditions;Inert atmosphere;Baeyer-Villiger Ketone Oxidation;
Steps:
General procedure for cyclic ketones oxidation
General procedure: The ketone (1.5 mmol) and silyl peroxide (2.25 mmol for reactions with bis(silyl) peroxides and 3 mmol for reactions with t-butyl silyl peroxides) were added dropwise at 0 °C into the freshly synthesised [hmim][AlxCly] (2 mmol for reactions with bis(silyl) peroxides and 3 mmol for reactions with t-butyl silyl peroxides). Next, the solution was stirred under a nitrogen atmosphere at room temperature for 1-24 h (depending on the reaction rate). After this time, 5 ml of water was added to the post-reaction mixture, and the water phase was extracted with methylene chloride or diethyl ether (10× 5 ml). The organic layer was washed with 5 ml of water, dried over anhydrous MgSO4, filtered, concentrated in a vacuum (50 °C, 100 mbar) and purified by column chromatography (hexane:ethyl acetate (4:1)) when necessary. All products were characterised by comparison of their NMR spectra (see Supplementary data) with authentic samples [20].
References:
Baj, Stefan;S?upska, Roksana;Chrobok, Anna;Drozdz, Agnieszka [Journal of Molecular Catalysis A: Chemical,2013,vol. 376,p. 120 - 126]
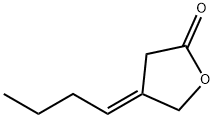
137669-51-3
0 suppliers
inquiry

22530-99-0
9 suppliers
inquiry
82918-72-7
0 suppliers
inquiry

22530-99-0
9 suppliers
inquiry