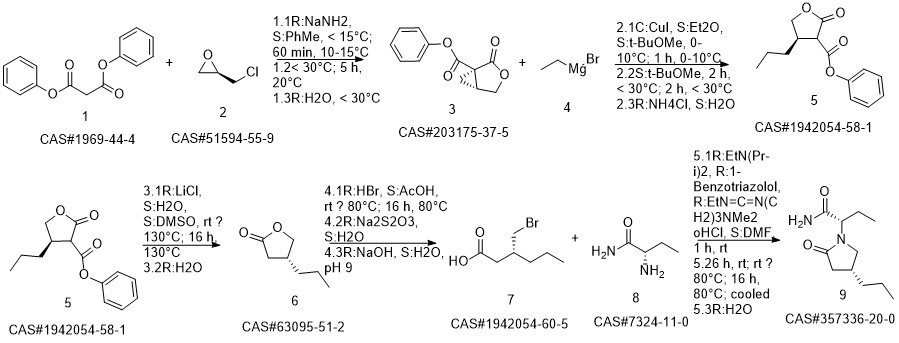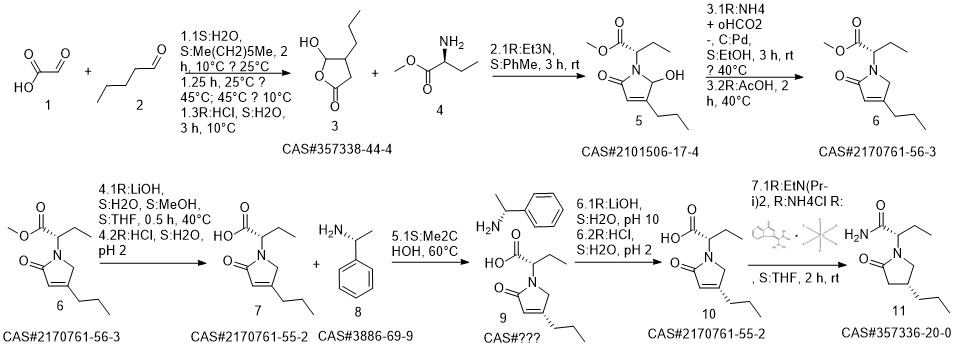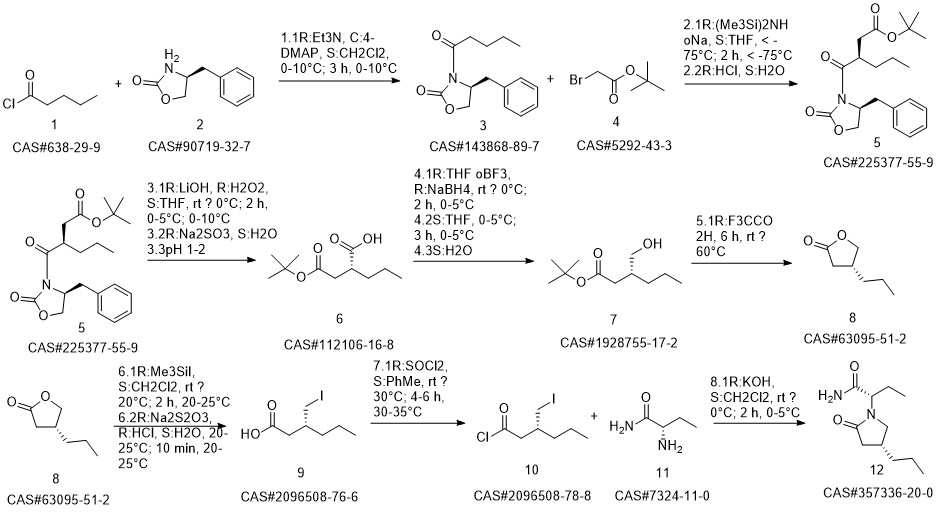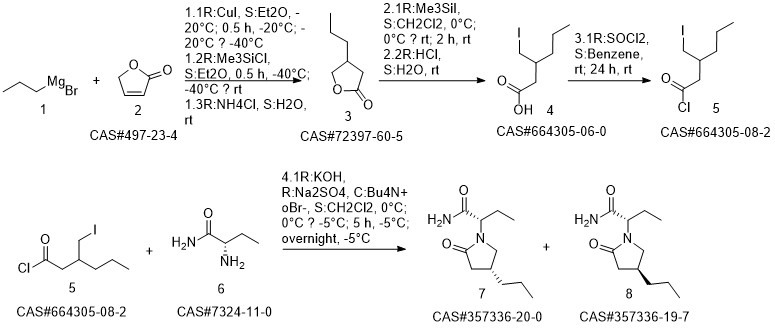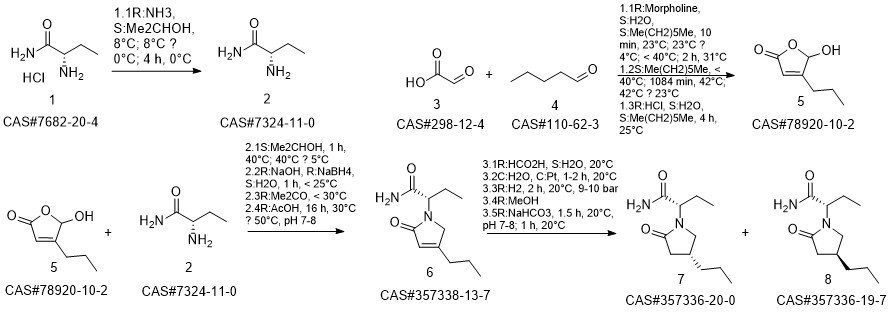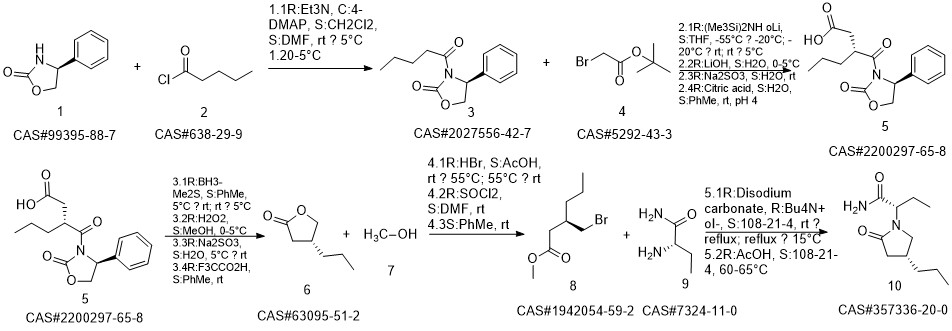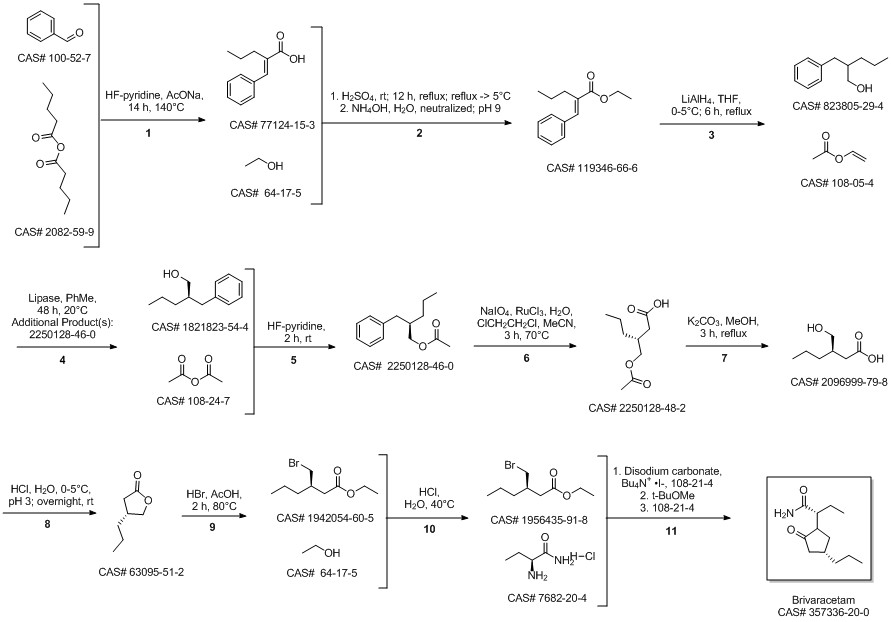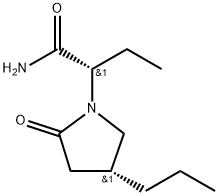
Brivaracetam synthesis
- Product Name: Brivaracetam
- CAS Number:357336-20-0
- Molecular formula:C11H20N2O2
- Molecular Weight:212.29
Reference: Qiu S, Tehrani KA, Sergeyev S, Bultinck P, Herrebout W, Mathieu B. Stereochemistry of the Brivaracetam Diastereoisomers. Chirality. 2016 Mar;28(3):215-25. doi: 10.1002/chir.22558. Epub 2016 Jan 6. PubMed PMID: 26740317.
Ciceri, Samuele; Grisenti, Paride; Elahi, Shahrzad Reza; Ferraboschi, Patrizia. A new chemoenzymatic synthesis of the chiral key intermediate of the antiepileptic brivaracetam. Molecules.Volume 23. Issue 9.Pages 2206/1-2206/11. 2018.
357338-13-7
36 suppliers
inquiry

357336-20-0
303 suppliers
$75.00/10mg
Yield:357336-20-0 99.3%
Reaction Conditions:
with (R)-(-)-5,5'-bis[di(3,5-ditert-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole;copper chloride (I);sodium tertiary butoxide in toluene at -40; for 24 h;Inert atmosphere;
Steps:
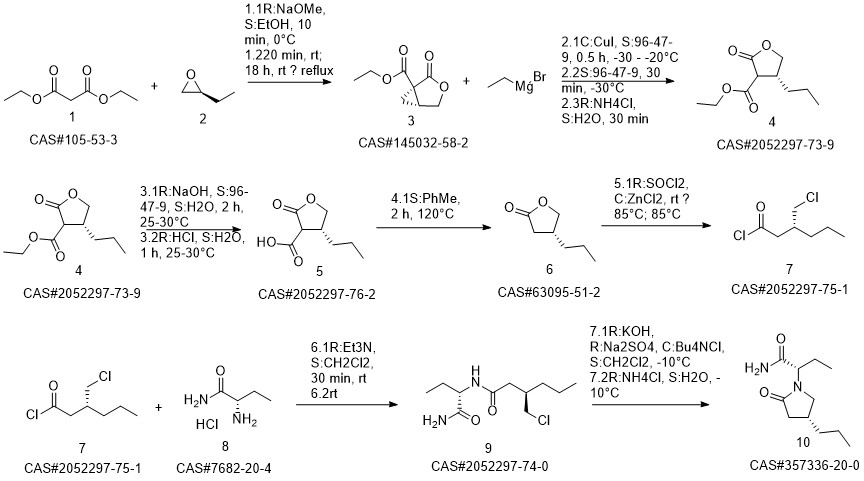
1
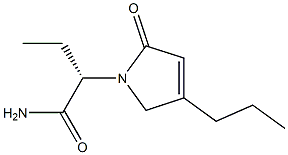
(2S) -2- ((4R) -2-oxo-4-n-propyl-1-pyrrolidinyl) butanamide,Which comprises the following steps: adding 430 mg of cuprous chloride, 420 mg of sodium tert-butoxide, 120 mg of (R) -DTBM-SEGPH0S to the reaction vessel under the protection of nitrogen And 240 ml of toluene, stirred for 20 min; then the temperature was adjusted to -40 ° C, 60 g of the starting material was added,(2S) - (2-oxo-4-propyl-2,5-dihydro-1-pyrrolyl) butanamide; and the reaction mixture was added 40 ml of polymethylhydrogensiloxane (S0 0.67 mil of polymethylhydrogensiloxane) and then incubated at a temperature of -40 ° C After 24 hours of reaction, the temperature was adjusted to 0 ° C, then 100 ml of saturated sodium bicarbonate solution and 150 ml of ether were added, And then stirring l0h; then, the use of ether on the reaction material extraction 2 times, combined with 2 extracted organic layer, and then there will beThe machine bed is dried, filtered, concentrated to obtain the product. 58 g of crude product was weighed and then recrystallized using isopropyl ether, 49 g of product was obtained, and the product after recrystallization was detected by high performance liquid chromatography. The detection conditions were: 0D-HColumn, n-hexane: isopropanol (90:10) as mobile phase, tassel is 1.01111 / 1 ^ 11, detection wavelength is 210 brain, detected by 4(R) type chiral material (i.e., (2S) -2- ((4R) -2-oxo-4-n-propyl-1-pyrrolidinyl) butanamide) 99.3%.
References:
CN104892483,2017,B Location in patent:Paragraph 0029-0030