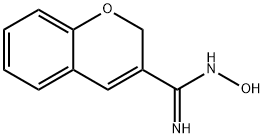
BRN 5521261 synthesis
- Product Name:BRN 5521261
- CAS Number:84748-20-9
- Molecular formula:C10H10N2O2
- Molecular Weight:190.2

57543-66-5
63 suppliers
inquiry

84748-20-9
9 suppliers
inquiry
Yield:84748-20-9 87%
Reaction Conditions:
with hydroxyamino hydrochloride;triethylamine in ethanol at 80; for 2 h;
Steps:
Synthesis of N′-hydroxy-2H-chromene-3-carboximidamide(3a)
A mixture of 2H-chromene-3-carbonitrile (intermediate 2a)(4 g, 25.4 mmol), hydroxylamine hydrochloride (3.2 g, 46.0mmol) and 10 ml of triethylamine were refluxed for 2 h inethanol. After the reaction, ethanol and excesses of triethylaminewas removed under reduced pressure. A lightyellow solid was obtained to which water (20 ml) wasadded and the solid precipitate was collected by filtration,washed with water and dried at 50 °C to furnish amidoxime(3a) as a white solid (4.2 g, 87% yield).N′-hydroxy-2H-chromene-3-carboximidamide (3a)White solid; m.p.: 200 °C, Rf 0.1 (20% EtoAc/hexane); IR(KBr): λmax 3400 (OH), 3340 (NH2), 1515 (C=N) cm-1;1H-NMR (CDCl3, 400MHz): δ 9.96 (1H, s, -OH),7.10-7.17 (2H, m, H-5,7), 7.00 (1H, s, H-4), 6.90 (1H, ddd,J = 1.0, 7.2, 8.5 Hz, H-6), 6.80 (1H, d, J = 8.0 Hz, H-8),5.66 (2H, s, NH2), 4.89 (2H, d, J = 1.2 Hz, OCH2); 13CNMR(CDCl3, 100.6 MHz): δ 153.6 (CN), 148.7 (C, C-8a),129.3 (CH, C-7), 127.2 (CH, C-5), 125.1 (C, C-3), 122.0(C, C-4a), 121.5 (CH, C-6), 120.4 (CH, C-4), 115.6 (CH, C-8), 63.7 (CH2, C-2); ESIMS m/z 191[M+].
References:
Kumar, K. Santosh;Daniel;Kaki, Shiva Shanker;Rao, Ch. Prasad;Krupadanam, G.L. David [Medicinal Chemistry Research,2016,vol. 25,# 10,p. 2179 - 2186]

90-02-8
444 suppliers
$9.00/1g

84748-20-9
9 suppliers
inquiry