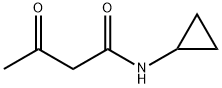
Butanamide, N-cyclopropyl-3-oxo- (9CI) synthesis
- Product Name:Butanamide, N-cyclopropyl-3-oxo- (9CI)
- CAS Number:110262-87-8
- Molecular formula:C7H11NO2
- Molecular Weight:141.17
Yield:110262-87-8 87%
Reaction Conditions:
in tetrahydrofuran at -5 - 0; for 1 h;
Steps:
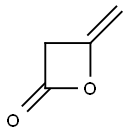
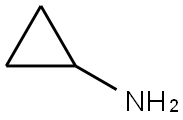
AJ AJ. N-Cyclopropyl-3-oxobutanamide
AJ. N-Cyclopropyl-3-oxobutanamide
26 ml (0.38 mmol) cyclopropylamine was added dropwise to a solution of 30 g of diketene (0.36 mol) in 300 ml tetrahydrofuran at -5 to 0° C.
After 1 h stirring at 0° C. no more starting material was detected by thin layer chromatography.
The reaction mixture was evaporated and the residue purified by column chromatography.
This gave 43.83 g (0.31 mol, 87% yield) of a white solid.
IR (neat, cm-1): 3266, 3082, 3017, 2953, 2922, 1724, 1666, 1636, 1450, 1421, 1358, 1338, 1221, 1193, 1161, 1014.
LC-MS: 140 (M-H).
1H-NMR (CDCl3, 400 MHz): δ [ppm]=7.2 (1H), 3.4 (2H), 2.7 (1H), 2.2 (3H), 0.7 (2H), 0.5 (2H).
References:
US2013/109662,2013,A1 Location in patent:Paragraph 0385; 0386; 0387; 0388; 0389