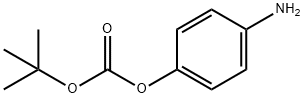
Cabozantinib impurity 4 synthesis
- Product Name:Cabozantinib impurity 4
- CAS Number:95932-39-1
- Molecular formula:C11H15NO3
- Molecular Weight:209.24
13303-10-1
8 suppliers
inquiry

95932-39-1
16 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen;palladium in ethyl acetate at 20; for 24 h;
Steps:
16.1
Step 1: Syntheses of 4-Aminophenyl t-butyl carbonate [Show Image] 4-nitrophenol (2 g, 14.37 mmol) was dissolved in dichloromethane (25 ml), added with di-t-butyl dicarbonate (3.76 g, 17.25 mmol) and 4-dimethylaminopyridine (2.28 g, 18.68 mmol). The resulting mixture was stirred for 10 hours at room temperature and poured into water. After extraction with chloroform, the organic layer was washed with brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was dissolved in ethyl acetate (30 ml), and 10%-palladium (Pd) (300 mg) was added. The mixture was then stirred uner hydrogen gas for one day at room temperature. Once the reaction was completed, 10%-palladium (Pd) was removed by celite-filter and the filtrate was concentrated to dryness. The residue was purified by flash column chromatography (hexane : ethyl acetate = 3 : 1) to obatin title compound (2.7 g, yield:90%, white solid). 1H NMR(400MHz, CDCl3) δ 6.94(d, J=4.4Hz, 2H), 6.64(d, J=4.4Hz, 2H) 3.62(br, 2H), 1.54(s, 9H)
References:
EP2364983,2011,A2 Location in patent:Page/Page column 34-35
123-30-8
605 suppliers
$10.00/5g
404586-94-3
8 suppliers
$838.25/5G

95932-39-1
16 suppliers
inquiry
![Carbonic acid, 4-[[(1,1-dimethylethoxy)carbonyl]amino]phenyl 1,1-dimethylethyl ester](/CAS/20200515/GIF/95932-40-4.gif)
95932-40-4
0 suppliers
inquiry

24424-99-5
825 suppliers
$13.50/25G

123-30-8
605 suppliers
$10.00/5g

95932-39-1
16 suppliers
inquiry
![Carbonic acid, 4-[[(1,1-dimethylethoxy)carbonyl]amino]phenyl 1,1-dimethylethyl ester](/CAS/20200515/GIF/95932-40-4.gif)
95932-40-4
0 suppliers
inquiry

24424-99-5
825 suppliers
$13.50/25G

95932-39-1
16 suppliers
inquiry
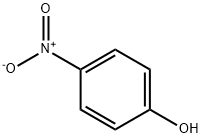
100-02-7
5 suppliers
$11.00/5G

95932-39-1
16 suppliers
inquiry