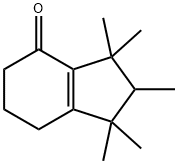
CASHMERAN synthesis
- Product Name:CASHMERAN
- CAS Number:33704-61-9
- Molecular formula:C14H22O
- Molecular Weight:206.32
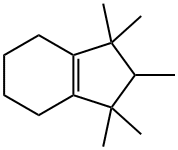
33704-59-5
2 suppliers
inquiry

33704-61-9
147 suppliers
$35.84/25G
Yield:-
Reaction Conditions:
Stage #1:2,3,4,5,6,7-hexahydro-1,1,2,3,3-pentamethyl-1H-indene with oxygen;cobalt-naphthenate catalyst (ALDRICH, 6 wt percent Co) in mineral spirits;water at 125; for 17 h;
Stage #2: with oxygen;Au-CeO2 catalyst in mineral spirits;water at 125; for 24 h;Product distribution / selectivity;
Steps:
2
Example 2; In a three-necked-round flask, 37 mg cobalt-naphthenate catalyst (ALDRICH, 6 wt % Co in mineral spirits) (Co-Naph, hereafter) were added to 14 g of an olefin/paraffin mixture containing 60 weight % 4,5,6,7-tetrahydro-1,1,2,3,3-pentamethylindane (THPMI hereafter), being the remaining fully saturated or aromatic derivatives from the previous compound, and 350 mg distilled water. The contents were heated up to 125° C. and magnetically stirred at 1000 rpm. When the desired temperature was reached (after c.a. 30 sec), air was fed at 0.66 mL/s. The progress of the reaction was followed by taking samples at regular periods and analyzed by GC/MS. N-hexadecane was used as the external standard. Peroxides were determined by adding a triphenylphosphine solution in acetone to the reaction sample. After 17 hours of reaction c.a. 56 mol % THPMI conversion was obtained, 26 mol % 1,2,3,5,6,7-hexahydro-1,1,2,3,3-pentamethyl-4H-inden-4-one and 21 mol % 4,5,6,7-tetrahydro-1,1,2,3,3-pentamethyl-4-indanol. This part comprises the first stage of the process in accordance with the earlier description of the invention. In the second stage of the process, 3 g of the above resulting mixture was directly taken, without further after-treatment, and 100 mg Au-CeO2 catalyst, prepared according to Example 1, were added. The contents were heated up to 125° C. and magnetically stirred at 1000 rpm. When the desired temperature was reached (after c.a. 30 sec), air was fed at 0.66 mL/s. The progress of the reaction was followed by taking samples at regular periods and analyzed by GC/MS. N-hexadecane was used as the external standard. Once again, peroxides were determined by adding a triphenylphosphine solution in acetone to the reaction sample. After 24 hours of reaction and the yield of 1,2,3,5,6,7-hexahydro-1,1,2,3,3-pentamethyl-4H-inden-4-one was 47 mol % with less than 1 mol % yield to 4,5,6,7-tetrahydro-1,1,2,3,3-pentamethyl-4-indanol, being the remaining polyoxygenated by-products.
References:
Navarro, Onofre Casanova;Canós, Avelino Corma;Jornet, Sara Iborra US2011/71320, 2011, A1 Location in patent:Page/Page column 4-5
