
Caspase-3/7 Inhibitor I synthesis
- Product Name:Caspase-3/7 Inhibitor I
- CAS Number:220509-74-0
- Molecular formula:C14H16N2O5S
- Molecular Weight:324.35
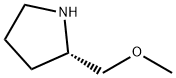
63126-47-6
165 suppliers
$36.70/250mg
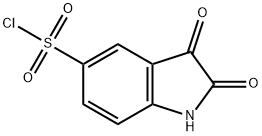
132898-96-5
35 suppliers
$55.00/2mg

220509-74-0
15 suppliers
inquiry
Yield:220509-74-0 93%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran;chloroform at 0; for 1.5 h;
Steps:
1.2 8-{[(2S)-2-(Methoxymethyl)pyrrolidin-1-yl]sulfonyl}-3,4-dihydropyrimido[1,2-a]indol-10(2H)-one
Step 2: 5-{[(2S)-2-(Methoxymethyl)pyrrolidin-1-yl]sulfonyl}-1H-indole-2,3-dione To a cold suspension of 2,3-dioxo-2,3-dihydro-1H-indole-5-sulfonylchloride (5.28 g, 21.5 mmol) in a 1:1 mixture of THF:CHCl3 (254 mL) was added drop-wise via syringe pump a solution of (S)-(+)-2-(methoxymethyl)-pyrrolidine (3.45 mL, 28.0 mmol, 1.3 eq) (Aldrich) and N,N-diisopropylethylamine (7.49 mL, 43 mmol, 2 eq) in CHCl3 (42 mL) over a period of 70 minutes under a dry N2 atmosphere with cooling in an ice bath. After stirring an additional 20 minutes the mixture was concentrated. The residue was flash chromatographed (Biotage KP silica gel, 98/2 CH2Cl2/CH3OH) to give the title compound as a dark greenish-yellow foam (6.48 g. 93% yield). NMR (400 MHz, DMSO-d6): consistent MS: (API-ES-) m/z 323[M-H].
References:
US2005/250798,2005,A1 Location in patent:Page/Page column 21
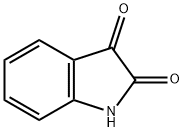
91-56-5
442 suppliers
$5.00/25g

220509-74-0
15 suppliers
inquiry
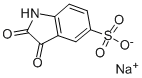
80789-74-8
15 suppliers
inquiry

220509-74-0
15 suppliers
inquiry

916049-16-6
5 suppliers
inquiry

220509-74-0
15 suppliers
inquiry
69610-40-8
386 suppliers
$6.00/5g

220509-74-0
15 suppliers
inquiry