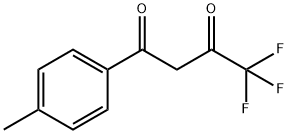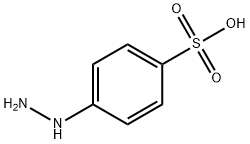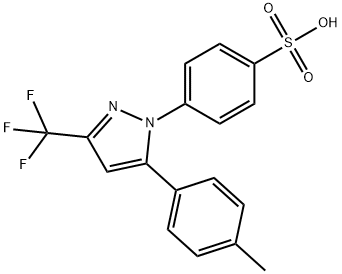
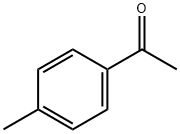
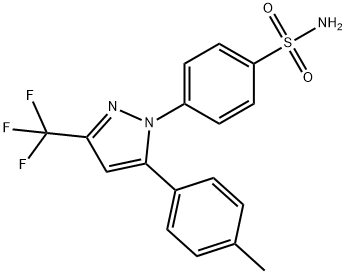
Celecoxib Impurity 22 synthesis
- Product Name:Celecoxib Impurity 22
- CAS Number:921617-76-7
- Molecular formula:C17H13F3N2O3S
- Molecular Weight:382.36
Yield:921617-76-7 82%
Reaction Conditions:
with hydrogenchloride in ethanol;water at 20; for 8 h;Heating / reflux;
Steps:
2
To a stirred suspension of p-hydrazino-benzenesulfonic acid (42 g, 0.223 mol) in ethanol (450 ml) 6N hydrochloric acid (74 ml, 0.446 mol) was added at room temperature, followed by addition of 4,4,4-trifluoro-l-(4-methyl-phenyl)-butane-l,3- dione (51.45 g, 0.223 mol). The obtained suspension was refluxed for 8 h, then concentrated in vacuo. The residue was dissolved in water (300 ml) and extracted with ethyl acetate (2 x 200 ml). The combined organic layers were washed with water (1 x 100 ml) and brine (1 x 100 ml), dried over MgSO4, decolorized, filtered and concentrated in vacuo. The obtained crystalline product was recrystallized from diisopropyl ether (300 ml) to yield 70.12 g (82 %) of the title compound.
References:
WO2007/12906,2007,A1 Location in patent:Page/Page column 15
122-00-9
681 suppliers
$5.00/25g

921617-76-7
25 suppliers
inquiry
169590-42-5
855 suppliers
$5.00/250mg

921617-76-7
25 suppliers
inquiry