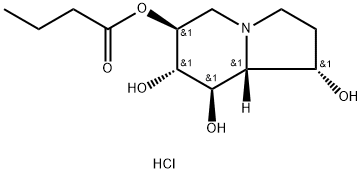
Celgosivir (hydrochloride) synthesis
- Product Name:Celgosivir (hydrochloride)
- CAS Number:141117-12-6
- Molecular formula:C12H22ClNO5
- Molecular Weight:295.76
79831-76-8
131 suppliers
$33.00/5mg

141-75-3
356 suppliers
$12.32/25G

141117-12-6
27 suppliers
inquiry
Yield: 77.4%
Reaction Conditions:
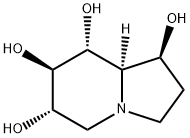
Stage #1:(1S,6S,7R,8R,8aR)-2,6,7,8-tetrahydroxy-indolizidine with bis(tri-n-butyltin)oxide in toluene for 3.33 h;Heating / reflux;
Stage #2:butyryl chloride in toluene at -13 - -8; for 0.166667 h;
Stage #3: with hydrogenchloride in ethanol;isopropyl alcohol;toluene at 18 - 30;
Steps:
2 Example 2; General Procedure for Preparinq 6-O-Butyrylcastanospermine [1S-(1α,6β,7α,8β,8aβ)]-octahydro-1,6,7,8-indolizinetetrol 6-butanoate HCl Two Liter Scale with Toluene as Solvent.
A 2 liter, three-neck flask equipped with a mechanical stirrer, thermometer and a Dean-Stark trap fitted with a condenser bearing a nitrogen bubbler is charged with castanospermine (20.00 g, 0.106 mol), bis(tributyltin) oxide (115 mL) and toluene (600 mL). The mixture is heated to reflux and water is collected in the Dean-Stark trap. After 3.33 hours the heating is stopped and the reaction mixture is cooled to 20C. The Dean-Stark trap is removed from the above reaction and is replaced with a Claisen adapter fitted.with a nitrogen bubbler and a pressure equalizing addition funnel. The reaction mixture is cooled to -13C and butyryl chloride (20.20 g, 0.190 mol) is added slowly over 10 minutes, maintaining the temperature between -13C and -8C. The reaction mixture is then allowed to warm to room temperature. The above reaction mixture is diluted with ethanol 3C (650 mL, ethanol denatured with 5% isopropanol) and treated with anhydrous hydrogen chloride (approximately 18.00 g, 0.494 mol). The reaction temperature rises from 18C to 30C during the hydrogen chloride addition. The reaction mixture is seeded with several crystals of 6-O-butyrylcastanospermine hydrochloride to produce a slurry of a white solid. The slurry is allowed to stir at room temperature overnight and then is cooled with an ice bath and stirred for 2 hours. The white solid is collected by filtration, air dried, washed with heptane (100 mL, mixed isomers), air dried and dried under vacuum at room temperature to provide the title compound (24.26 g, 77.4%).
References:
Aventis Pharmaceuticals Inc. EP983270, 2004, B1 Location in patent:Page 8