
Centhaquine synthesis
- Product Name:Centhaquine
- CAS Number:57961-90-7
- Molecular formula:C22H25N3
- Molecular Weight:331.45
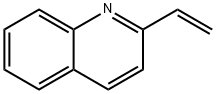
772-03-2
53 suppliers
$65.00/250mg
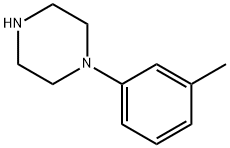
41186-03-2
114 suppliers
$28.00/5g

57961-90-7
31 suppliers
inquiry
Yield:57961-90-7 73%
Reaction Conditions:
with acetic acid in ethanol; for 24 h;Reflux;
Steps:
Synthesis and characterization of centhaquin (free base):
A mixture of 2-vinyiquinoline (1) (5.0 g, 32.2 mmol, 98.5%) and l-(3-methylphenyl)piperazine (2) (5.68 g, 32.2 mmol, 99.0%) in absolute ethyl alcohol (150 ml) and glacial acetic acid (3.5 ml) was stirred at reflux for 24 hours in a round bottom flask. The reaction mixture was concentrated in vacua, diluted with water (150 ml) and treated with 10% aqueous NaOH (150 ml). The residue was extracted with ethyl acetate (4 x 125 ml), dried with anhydrous Na2SO4, and concentrated under reduced pressure to yield a crude product which was purified by column chromatography using silica gel (100-200 mesh) with ethyl acetate as an eluent. The resulting compound was recrystallized from hot hexane and filtered, to yield centhaquin as an off- white crystalline solid (7.75 g, 23.4 mmol, 73% yield); mp. 94-95°C; Rf 0.30 (100% ethyl acetate); 1H NMR (300 MHz, CDCl3): 8 8.07 (t, J= 7.5 Hz, 2 H), 7.78 (d, J= 7.8 Hz, 1 H),7.70 (t, J= 7.8 Hz, 1 H), 7.50 (t, J= 7.5 Hz, 1 H), 7.36 (d, J= 8.4 Hz, 1 H), 7.16 (t, J= 7.5 Hz, 1 H), 6.77 - 6.74 (m, 2 H), 6.69 (d, J= 7.2 Hz, 1 H), 3.26- 3.21 (m, 6 H), 2.97 - 2.92 (m,2 H), 2.76 - 2.73 (m, 4 H), 2.32 (s, 3 H);HRMS (ESI) m/z 332.2121 [M+1]+ (calcd for C22H26N3 332.2122); Anal. (C22H25N3) C, H, N.
References:
WO2014/35446,2014,A1 Location in patent:Paragraph 0063
91-63-4
270 suppliers
$23.00/25g

57961-90-7
31 suppliers
inquiry
63487-23-0
0 suppliers
inquiry

57961-90-7
31 suppliers
inquiry

41186-03-2
114 suppliers
$28.00/5g

57961-90-7
31 suppliers
inquiry