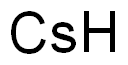
Cesium synthesis
- Product Name:Cesium
- CAS Number:7440-46-2
- Molecular formula:Cs
- Molecular Weight:132.91
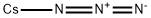
22750-57-8
23 suppliers
inquiry
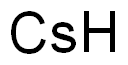
7440-46-2
83 suppliers
$76.96/1g
Yield:7440-46-2 90%
Reaction Conditions:
in neat (no solvent);byproducts: caesium nitride, caesium silicate; CsN3 was decomposed at 390 °C forming Cs and a yellowish gray residue (contains ca. 70% Cs-nitride beside Cs-silicate and not-reacting Cs-azide);;
References:
Suhrmann, R.;Clusius, K. [Zeitschrift fur anorganische Chemie,1926,vol. 152,p. 55 - 55]
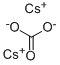
534-17-8
626 suppliers
$5.00/5g
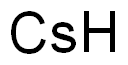
7440-46-2
83 suppliers
$76.96/1g
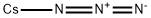
22750-57-8
23 suppliers
inquiry

7727-37-9
111 suppliers
$323.00/56l
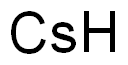
7440-46-2
83 suppliers
$76.96/1g
7789-16-4
0 suppliers
inquiry
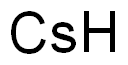
7440-46-2
83 suppliers
$76.96/1g