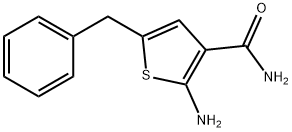
CHEMBRDG-BB 3007468 synthesis
- Product Name:CHEMBRDG-BB 3007468
- CAS Number:383382-37-4
- Molecular formula:C12H12N2OS
- Molecular Weight:232.3
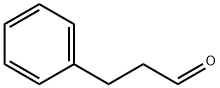
104-53-0
268 suppliers
$6.00/5g
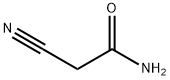
107-91-5
539 suppliers
$5.00/10g

383382-37-4
14 suppliers
$58.00/100mg
Yield:383382-37-4 53%
Reaction Conditions:
with sulphur;triethylamine in DMF (N,N-dimethyl-formamide) at 20;
Steps:
6.a Example 6; Preparation of 5-Benzyl-2-ureido-thiophene-3-carboxylic acid amide; 2-Amino-5-benzyl-thiophene-3-carboxylic acid amide trifluoroacetate
Triethyl amine (0.28 mL, 2.0 mmol) was added dropwise to a mixture of 2- cyano-acetamide (0.168 g, 2.0 mmol), sulfur (0.064 g, 2.0 mmol) and 3-phenyl propionaldehyde (0.29 g, 2.0 mmol) in DMF (8 mL)]and resulting reaction mixture was stirred at room temperature overnight. The reaction mixture was then filtered and purified utilizing a Gilson preparative HPLC to give the above titled compound as light yellow solid (0.25 g, 1.06 mmol, 53 % yield). LC-MS [M+H]+ m/z 233.
References:
WO2003/104218,2003,A1 Location in patent:Page 17