
CHEMBRDG-BB 3018393 synthesis
- Product Name:CHEMBRDG-BB 3018393
- CAS Number:58621-54-8
- Molecular formula:C11H12O2
- Molecular Weight:176.21
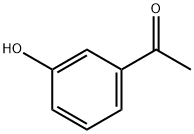
121-71-1
634 suppliers
$5.00/5g

106-95-6
424 suppliers
$10.00/5g

58621-54-8
13 suppliers
$45.00/100mg
Yield:58621-54-8 100%
Reaction Conditions:
with potassium carbonate in acetone at 20;Reflux;
Steps:
154.154-a-1
Example 154; Preparation of 3-((4-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-2-propylphenoxy)pyridin-2-yl)methyl)-5-methyl-5-(2-methyl-2,3-dihydrobenzofuran-6-yl)imidazolidine-2,4-dione; 154-a-1) Preparation of 1-(3-(allyloxy)phenyl)ethanone; 3-Hydroxyacetophenone (1.36 g, 10.0 mmol) was dissolved in acetone (50 mL), then added with potassium carbonate (2.76 g, 20.0 mmol) and allylbromide (1.27 mL, 15.0 mmol) at room temperature, and heated to reflux for 2 hours. The reaction solution was cooled to room temperature, added with ethyl acetate, and washed with water and brine. The organic layer was dried over anhydrous sodium sulfate and concentrated in vacuo. The obtained residue was purified using silica-gel column chromatography (n-hexane/ethyl acetate=10/1), and 1-(3-(allyloxy)phenyl)ethanone (1.76 g, 100%) was obtained as a pale yellow oil.1H-NMR (CDCl3) δ: 2.59 (3H, s), 4.60 (2H, d, J=5.4 Hz), 5.31 (1H, dd, J=1.5, 10.5 Hz), 5.43 (1H, dd, J=1.5, 17.3 Hz), 6.02-6.11 (1H, m), 7.13 (1H, d, J=8.3 Hz), 7.37 (1H, t, J=7.8 Hz), 7.50-7.55 (1H, m).
References:
US2010/48610,2010,A1 Location in patent:Page/Page column 86
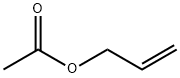
591-87-7
184 suppliers
$10.00/10g

121-71-1
634 suppliers
$5.00/5g

58621-54-8
13 suppliers
$45.00/100mg
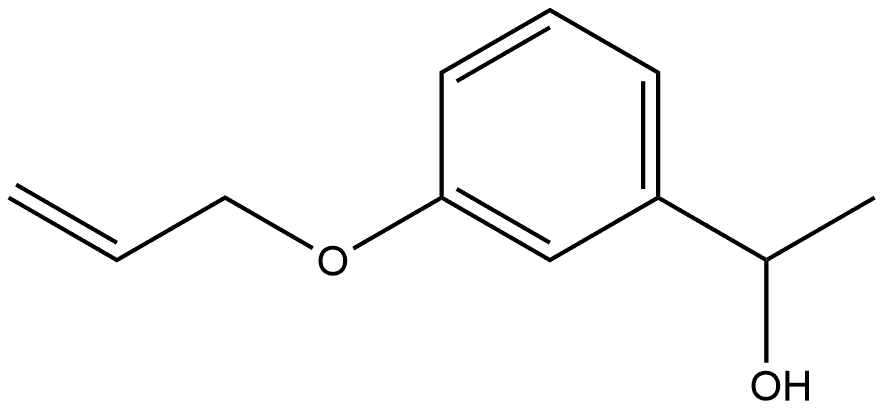
79250-49-0
0 suppliers
inquiry

58621-54-8
13 suppliers
$45.00/100mg
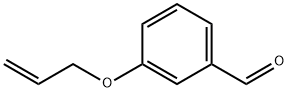
40359-32-8
47 suppliers
$31.00/250mg

58621-54-8
13 suppliers
$45.00/100mg
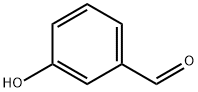
100-83-4
623 suppliers
$5.00/10g

58621-54-8
13 suppliers
$45.00/100mg