CHEMBRDG-BB 4004844 synthesis
- Product Name:CHEMBRDG-BB 4004844
- CAS Number:112086-55-2
- Molecular formula:C8H12N2O
- Molecular Weight:152.19

1336-21-6
698 suppliers
$14.56/250ML
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

112086-55-2
13 suppliers
$121.00/250 mg
Yield: 0.354 g (20.1%)
Reaction Conditions:
with sodium chloride in methanol
Steps:
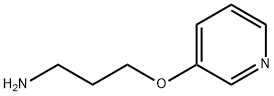
10 3-(3-Pyridyloxy)propylamine
3-(3-Pyridyloxy)propylamine The 3-chloro-1-(3-pyridyloxy)propane (1.98 g, 11.6 mmol) was dissolved in methanol (25 mL) and added to concentrated ammonium hydroxide solution (29.7%, 14.8 M, 55 mL) in a heavy-walled glass pressure-tube apparatus. The tube was sealed and the mixture was stirred and heated at 100° C. (oil bath temperature) for 6 h. After cooling, the mixture was concentrated by rotary evaporation. Saturated NaCl solution (10 mL) was added to the residue, and the solution (pH 6) was extracted with ether (3*25 mL) to remove impurities. The aqueous layer was basified to pH 10 with 10% NaOH solution, and the mixture was extracted with chloroform (4*25 mL). The combined chloroform extracts were dried (Na2SO4), filtered, and concentrated by rotary evaporation to a residue that was dried briefly under high vacuum to give 0.354 g (20.1%) of an oil.
References:
DULL, GARY MAURICE;WAGNER, JARED MILLER;HADIMANI, SRISHAIKUMAR BASAWANNAPPA;CONSILVIO, MICHAEL B. US2001/14691, 2001, A1
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

112086-55-2
13 suppliers
$121.00/250 mg