
CHEMBRDG-BB 4017853 synthesis
- Product Name:CHEMBRDG-BB 4017853
- CAS Number:915925-45-0
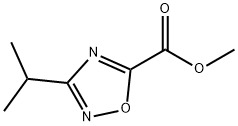
- Molecular formula:C6H10N2O2
- Molecular Weight:142.16
1132841-11-2
0 suppliers
inquiry

915925-45-0
23 suppliers
$65.00/100mg
Yield:915925-45-0 65%
Reaction Conditions:
Stage #1: C7H10N2O3with lithium borohydride in tetrahydrofuran at 20; for 15 h;
Stage #2: with water in tetrahydrofuran;
Steps:
16.B
Step B: 3-isopropyl-5-(hydroxymethyl')oxadia2ple Methyl 3-isopropyl-5-oxadiazole carboxylate (1.0 g, 4.67 mmol) obtained in Step A was dissolved in THF (10 mL) and LiBH4 (186 mg, 9.3 mmol) was added thereto, followed by stirring at room temperature for 15 hours. After the reaction was complete, water was gradually added, followed by extraction with EtOAc. The organic extract was dried over MgSO4 and distilled under reduced pressure. The residue was purified by column chromatography (EtOAc/n-Hex = 1/5) to afford the title compound (431 mg, 65%).
References:
WO2009/38411,2009,A2 Location in patent:Page/Page column 38