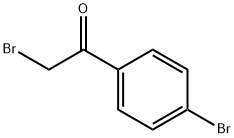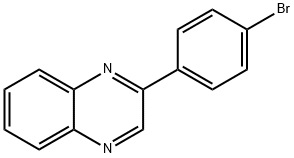
CHEMBRDG-BB 5128501 synthesis
- Product Name:CHEMBRDG-BB 5128501
- CAS Number:5021-45-4
- Molecular formula:C14H9BrN2
- Molecular Weight:285.14
Yield:5021-45-4 98%
Reaction Conditions:
with Bronsted acidic ionic liquid supported on nano silica in neat (no solvent) at 20; for 0.5 h;Green chemistry;
Steps:
General Procedure for the Synthesis of 2-Arylquinoxalines (3a-h)
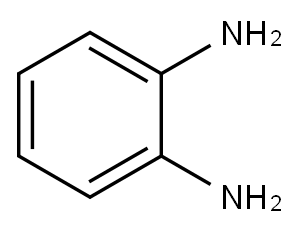
General procedure: In 25 ml round-bottomed flask, a mixture of 1,2-phenylendiamine (1 mmol), a-bromoacetophenone(1 mmol) and BAILnano-SiO2 catalyst (35 mol%) was preparedand the reaction mixture was stirred at room temperature for the time shown inTable 2. The progress of the reaction was monitored by TLC. After completion of the reaction, ethyl acetate (5 mL) was added and the catalyst was separated by simplefiltration. The residue was dried over anhydrous Na2SO4 and after evaporation ofsolvent the pure product was obtained by recrystallization from ethanol.
References:
Daragahi, Seyed Arash Hosseini;Mohebat, Razieh;Mosslemin, Mohammad Hossein [Organic Preparations and Procedures International,2018,vol. 50,# 3,p. 301 - 313]
3343-45-1
70 suppliers
$44.00/250mg
95-54-5
496 suppliers
$9.00/1g

5021-45-4
30 suppliers
$60.00/500mg

27200-79-9
107 suppliers
$47.00/250mg

95-54-5
496 suppliers
$9.00/1g

5021-45-4
30 suppliers
$60.00/500mg
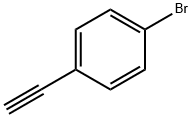
766-96-1
222 suppliers
$7.00/250mg

95-54-5
496 suppliers
$9.00/1g

5021-45-4
30 suppliers
$60.00/500mg
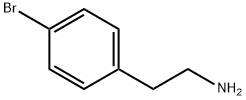
73918-56-6
234 suppliers
$20.00/1g

95-54-5
496 suppliers
$9.00/1g

5021-45-4
30 suppliers
$60.00/500mg