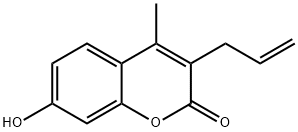
CHEMBRDG-BB 5223960 synthesis
- Product Name:CHEMBRDG-BB 5223960
- CAS Number:26481-13-0
- Molecular formula:C13H12O3
- Molecular Weight:216.23
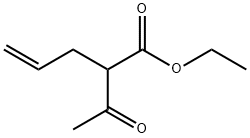
610-89-9
22 suppliers
$60.00/500mg
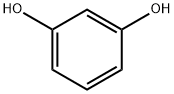
108-46-3
747 suppliers
$10.00/10g

26481-13-0
11 suppliers
$98.00/1g
Yield:26481-13-0 13%
Reaction Conditions:
with sulfuric acid in 1,4-dioxane at 0 - 20;Inert atmosphere;
Steps:
Synthesis of 3-allyl-7-hydroxy-4-methyl-2H-chromen-2-one (2):
This compound was prepared using Pechmann condensation as follows. A solutionof resorcinol (4.06 g, 36.90 mmol, 1 equiv.) and ethyl 2-acetylpent-4-enoate (1) (6.90 g,40.50 mmol, 1.1 equiv.) in dioxane (80 mL) was cooled to 0 C, followed by drop-wiseaddition of concentrated H2SO4 (98%, 19.60 mL, 405 mmol, 10 equiv.). The reaction mixturewas stirred at room temperature overnight. Dioxane was then evaporated under reducedpressure and the semi-solid mixture was added portion-wise to an ice-cold solution of KOH (40 g) in H2O (100 mL). The pH was adjusted to 13 with KOH and the resulting white solidwas filtered off. The filtrate was extracted with EtOAc (3 25 mL) and the combined organicextracts were dried over anhydrous Na2SO4 and evaporated under reduced pressure.The compound was purified by column chromatography (EtOAc/n-hexane, 1/1.5, v/v,dry loading) yielding a pale-yellow solid. Yield: 13%. 1H-NMR (400 MHz, DMSO-d6) 2.34(s, 3H, CH3), 3.30 (d, J = 6.0 Hz, 2H, Ar-CH2CHCH2), 4.98-5.06 (m, 2H, Ar-CH2CHCH2),5.84 (ddt, J = 16.3, 10.3, 6.0 Hz, 1H, Ar-CH2CHCH2), 6.70 (d, J = 2.4 Hz, 1H, Ar-H), 6.80 (dd,J = 8.7, 2.4 Hz, 1H, Ar-H), 7.63 (d, J = 8.7 Hz, 1H, Ar-H), 10.44 (s, 1H, OH); HRMS (ESI) m/zcalculated for C13H11O3 [M H] 215.0714, found 215.0707.
References:
?terman, Andrej;Gobec, Martina;Gobec, Stanislav;Mravljak, Janez;Proj, Matic;Rejc, Luka;Schiffrer, Eva Shannon;Sosi?, Izidor [Molecules,2021,vol. 26,# 2]