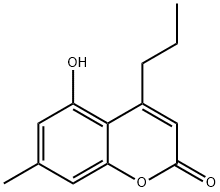
CHEMBRDG-BB 6147652 synthesis
- Product Name:CHEMBRDG-BB 6147652
- CAS Number:66346-53-0
- Molecular formula:C13H14O3
- Molecular Weight:218.25
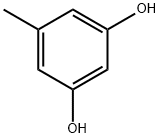
504-15-4
376 suppliers
$7.00/100mg
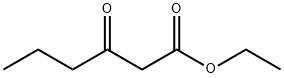
3249-68-1
237 suppliers
$6.00/5g

66346-53-0
14 suppliers
$46.00/1g
Yield:66346-53-0 29%
Reaction Conditions:
with sulfuric acid at 0 - 20; for 1.5 h;Pechmann Condensation;
Steps:
General procedure: Conc. H2SO4 (7.7 mL, 0.145 mol) was added to a mixture of 5-methylresorcinol (1) (18 g, 0.145 mol) and methyl 3-oxoheptanoate (25.2 g, 0.159 mol) at 0 °C. The brown slurry was stirred at room temperature for 1.5 h. H2O-hexane-EtOAc (110 mL; 5:5:1, v/v) was added to the reaction mixture and the resulting suspension was stirred at room temperature for 10 min. Precipitates were collected by filtration and the crude product was triturated with MeOH to give the title compound (2e) (21.9 g) as a pale yellow solid. The combined filtrate was extracted three times with EtOAc. Conc. H2SO4 (7.0 mL, 0.131 mol) was added to the combined organic layers and the resulting mixture was stirred at room temperature for 1 h. After addition of H2O, precipitates were collected by filtration and the crude product was triturated with MeOH to give the second crop (6.94 g) as a yellow solid.
References:
Cho, Nobuo;Kikuzato, Ko;Futamura, Yushi;Shimizu, Takeshi;Hayase, Hiroki;Kamisaka, Kikuko;Takaya, Daisuke;Yuki, Hitomi;Honma, Teruki;Niikura, Mamoru;Kobayashi, Fumie;Watanabe, Nobumoto;Osada, Hiroyuki;Koyama, Hiroo [Bioorganic and Medicinal Chemistry,2022,vol. 66,art. no. 116830] Location in patent:supporting information