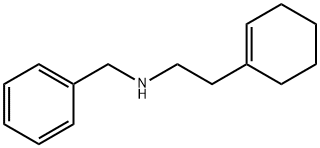
CHEMBRDG-BB 6610361 synthesis
- Product Name:CHEMBRDG-BB 6610361
- CAS Number:118647-00-0
- Molecular formula:C15H21N
- Molecular Weight:215.33

100-52-7
963 suppliers
$20.37/250g
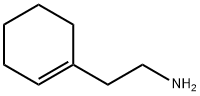
3399-73-3
272 suppliers
$13.00/5g

118647-00-0
8 suppliers
$65.00/50mg
Yield:118647-00-0 41%
Reaction Conditions:
Stage #1: benzaldehyde;2-(1-cyclohexenyl)ethylamine in methanol at 100; for 2 h;
Stage #2: with methanol;sodium tetrahydroborate at 60; for 6 h;
Steps:
13 B1 : N-benzyl-2-(cyclohex-1-en-1-yl)ethan-1-amine
2-(cyclohex-1-en-1-yl)ethan-1-amine (0.5 mmol) and benzaldehyde (0.55 mmol) were dissolved in 0.6 ml MeOH, heated at 100 °C for 2 hours; then the mixture was cooled, NaBH4 (0.5 mmol) was added and stirred for 4 hours. The mixture was heated for 2 hours at 60°C; 3 ml of methanol and 0.2 g of C-18 chromatographic phase were added, stirred for 2 hours, filtered, evaporated and dissolved in 0.5 ml of DMSO. The residue was purified by HPLC. Yield: 41 %. 1H NMR (400 MHz, DMSO-d6) d 9.73 (s, 2H), 7.61 (d, J = 7.6 Hz, 2H), 7.39 (d, J = 6.4 Hz, 3H), 5.46 (s, 1H), 4.07 (t, J = 5.6 Hz, 2H), 3.11 (s, 2H), 2.88 (d, J = 9.0 Hz, 2H), 2.36 (t, J = 8.3 Hz, 2H), 1.98 (s, 2H), 1.90 (d, J = 7.4 Hz, 2H), 1.60 (q, J = 5.7 Hz, 2H), 1.55 (q, J = 5.9 Hz, 2H). m/z=2l6.2
References:
WO2019/140265,2019,A1 Location in patent:Paragraph 00580

3399-73-3
272 suppliers
$13.00/5g

118647-00-0
8 suppliers
$65.00/50mg
931-49-7
63 suppliers
$11.00/250mg

100-46-9
488 suppliers
$5.00/5G

118647-00-0
8 suppliers
$65.00/50mg

108-94-1
551 suppliers
$12.00/50g

118647-00-0
8 suppliers
$65.00/50mg
6975-71-9
158 suppliers
$25.00/5g

118647-00-0
8 suppliers
$65.00/50mg