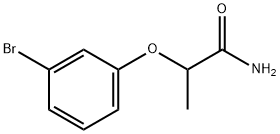
CHEMBRDG-BB 9070477 synthesis
- Product Name:CHEMBRDG-BB 9070477
- CAS Number:915923-02-3
- Molecular formula:C9H10BrNO2
- Molecular Weight:244.09

915923-02-3
12 suppliers
$46.00/1g
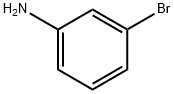
591-19-5
326 suppliers
$10.00/10g
Yield:591-19-5 65%
Reaction Conditions:
with potassium hydroxide in dimethyl sulfoxide at 140; for 4 h;Green chemistry;
Steps:
General Procedure A
General procedure: A reaction tube was charged with the 2-aryloxypropanamide (1.0 mmol), potassium hydroxide (112 mg, 2.0 mmol) and dimethyl sulphoxide (DMSO 4 mL). The mixture was heated at 140 C for 3-16 h. The reaction was monitored by TLC. After the reaction period, the reaction mixture was cooled, diluted with saturated brine and extracted with dichloromethane three times. The combined organic layers were washed with three portions of saturated brine and then dried over MgSO4 and filtered. Solvent was removed under reduced pressure. The product was purified through flash column chromatography on 300-400 mesh silica gel with petroleum ether/ethyl acetate as eluent
References:
Yu, Jianzhong;Zhang, Peizhi;Wu, Jun;Shang, Zhicai [Tetrahedron Letters,2013,vol. 54,# 24,p. 3167 - 3170] Location in patent:supporting information