
CHEMBRDG-BB 9071382 synthesis
- Product Name:CHEMBRDG-BB 9071382
- CAS Number:908518-19-4
- Molecular formula:C7H8BrNOS
- Molecular Weight:234.11
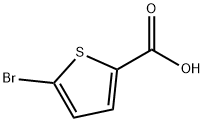
7311-63-9
335 suppliers
$5.00/250mg
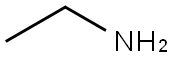
75-04-7
377 suppliers
$10.00/20mg

908518-19-4
13 suppliers
$46.00/1g
Yield:908518-19-4 67.8%
Reaction Conditions:
Stage #1: 5-bromothiophene-2-carboxylic acid;ethylamine in dichloromethane at 0; for 0.0833333 h;
Stage #2: with 4-methyl-morpholine;benzotriazol-1-ol in dichloromethane at 0 - 5; for 0.5 h;
Stage #3: with 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in dichloromethane at 0 - 20; for 13 h;
Steps:
1.2.I
A solution of amine (VI) (28.98 mmoles) in DCM (200 ml) was added to a stirred and cooled (0° C) solution of 5-bromo-2-thiophenecarboxylic acid (IV) (24.15 mmoles) in DCM (250 ml) and the mixture was stirred for 5 min. To the above mass HOBt (9.66 mmoles) and N-methylmorpholine (28.98 mmoles) were added and stirred for 30 min at 0- 50C. After 30 minutes EDC HCl (28.98 mmoles) was added and stirred for 3 h at 0° C and then at room temperature for 1O h. The mixture was diluted with water (100 ml) and organic layer was separated. The organic layer was washed with sodium bicarbonate solution and brine solution, dried over Na2SO4, distillation of solvent completely under reduced pressure to residue. To the residue hexane (50 ml) added and stirred for 30 min, filtered to get the desired compound (VIII).
References:
WO2009/137081,2009,A2 Location in patent:Page/Page column 35-36