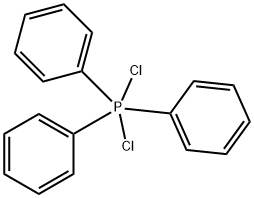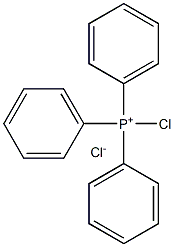
chloro(triphenyl)phosphonium chloride synthesis
- Product Name:chloro(triphenyl)phosphonium chloride
- CAS Number:19171-57-4
- Molecular formula:C18H15Cl2P
- Molecular Weight:333.1915
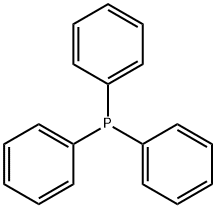
603-35-0
698 suppliers
$5.00/5G

19171-57-4
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with oxalyl dichloride in toluene at 20; for 0.5 h;Inert atmosphere;Schlenk technique;
Steps:
Oxalyl chloride (0.17 mL, 2.0 mmol 2 equiv) was added dropwise at roomtemperature to a solution of PTMP (10.0 mL, 0.11 M, 1.0 mmol in dry toluene,1 equiv), which was taken in a flame-dried degassed Young’s tube. The reactionwas allowed to stir for 30 min. 31P NMR was taken to identify the intermediatewhich shows full conversion of phosphine oxide into chlorophosphonium salt(CPS) 70.5 ppm. Excess oxalyl chloride was completely removed through adedicated cold trap connected to a Schlenk manifold for 60 min. Dry CH2Cl2(10 mL) was added to dissolve CPS again and the mixture was cooled to 82 Cusing an EtOAc/N2 mixture and ()-menthol (15 mL, 0.132 M, in dry toluene,2.0 equiv) was added dropwise to the mixture over 30 min. The reaction wasallowed to reach room temperature and was allowed to stir overnight at 0 C.31P NMR was taken to identify the intermediate which shows full conversion of(CPS) into diastereomeric alkoxyphosphonium salts (DAPS) at 66.6 and66.8 ppm. IPA (10 mL) was added dropwise to the mixture, which was thenrefluxed for 2 h. 31P NMR shows full conversion from DAPS into phosphineoxide. The solvent was removed and column chromatography was carried outon silica gel (EtOAc:cyclohexane 50:50) to separate excess menthol,neomenthyl chloride and menthol oxalate followed by (MeOH-CH2Cl2)yielding (R)-PTMPO as a white solid; 31P NMR 32.2 ppm (lit. 32.5 ppm)(0.22 g 88%). A portion of the sample (3 mg) was dissolved in 2 mL of HPLCsolvent (HPLC grade solvents purchased from Aldrich were used as supplied)and filtered through a PTFE syringe filter into a HPLC vial. High-performanceliquid chromatography was performed on an Agilent Technologies 1200 seriesconnected to a 6-column switcher. HPLC (CHIRALPAK IA column, 80:20heptane/EtOH, 1 mL/min): 81.4% ee Rt = 6.8 (S), 7.6 (R) min.
References:
Rajendran, Kamalraj V.;Kennedy, Lorna;O'Connor, Cormac T.;Bergin, Enda;Gilheany, Declan G. [Tetrahedron Letters,2013,vol. 54,# 51,p. 7009 - 7012]
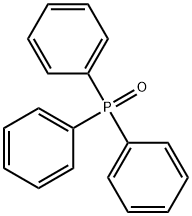
791-28-6
486 suppliers
$6.00/25g

19171-57-4
0 suppliers
inquiry
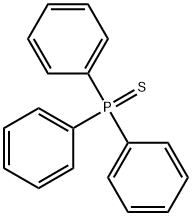
3878-45-3
137 suppliers
$8.00/5g

19171-57-4
0 suppliers
inquiry
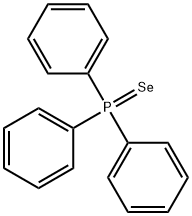
3878-44-2
75 suppliers
$79.00/5g

19171-57-4
0 suppliers
inquiry