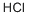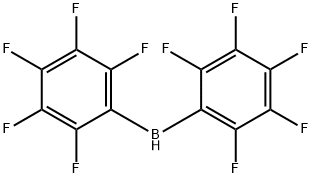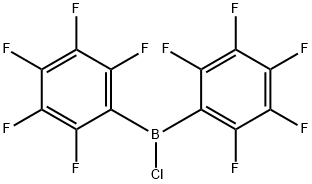
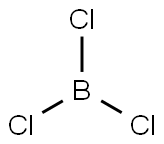
CHLOROBIS(PENTAFLUOROPHENYL)BORANE synthesis
- Product Name:CHLOROBIS(PENTAFLUOROPHENYL)BORANE
- CAS Number:2720-03-8
- Molecular formula:C12BClF10
- Molecular Weight:380.38
10294-34-5
257 suppliers
$28.00/25ML
801-79-6
5 suppliers
inquiry

2720-03-8
10 suppliers
inquiry

753-73-1
195 suppliers
$26.69/2g
Yield: 82% , 90%
Reaction Conditions:
in n-heptane at -70 - 105;
Steps:
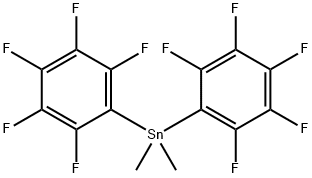
2 Example 2; Bis(pentafluorophenyl)boron Chloride, (C6f5)2BCl (compound 2)
A solution of dimethylbis(pentafluorophenyl)tin, Me2Sn(C6F5)2, (40.98 g, 84.9 mmol) in 30 ml of dry heptane was placed in a thoroughly dried 500 ml Schlenk tube equipped with a side-arm with a J-Young stopcock, a Teflon/rubber double 0-ring stopper and a stirrer bead. The solution was cooled to -70 C and a solution of BCl3 (85 ml of a 1.0 M solution in heptane, 85 mmol) was added via a syringe. The Schlenk tube was tightly sealed and the solution was allowed to warm up slowly to room temperature and stirred for 2.5 hours at RT, during which time some precipitation occurred. The Schlenk tube was then heated for 30 hours in an oil bath at 105 C. Some crystals may condense in the top part of the tube during this process. The reaction mixture was allowed to cool slowly to room temperature overnight, a large amount of Me2SnCl2 crystals precipitating out of the solution. The supernatant was transferred by means of a cannula to another thoroughly dried flask, after which the crystals were washed with hexane (2 x 20 ml), the hexane being combined with the supernatant. By drying the crystals, pure Me2SnCl2 was more than 90% recovered. After the volatile components had been stripped from the solution under reduced pressure, the off-white solid was briefly dried under vacuum to give 31.3 g of crude product, which was redissolved in the minimum volume of hexane. After filtration of the insoluble components (principally Me2SnCl2), the clear solution was cooled at -30 C overnight. The supernatant was removed through a cannula while the solution was still cold, and the crystals were dried under vacuum to give the pure compound, ClB(C6F5)2, (26.42 g, 82%) in the form of extremely air-sensitive and moisture-sensitive crystals. 11B NMR (80.25 MHz, CD2Cl2), δ 58.0 (br. s). 19F NMR (376.3 MHz, CD2Cl2), δ -129.5 (m, 4F, ortho), -145.5 (m, 2F, para), -161.6 (m, 4F, meta). 1.a). R. Duchateau, S. J. Lancaster, M. Thornton-Pett and M. Bochmann. Organometallics 1997, 16, 4995. b). M. Bochmann, S. J. Lancaster and O. B. Robinson. J. Chem. Soc. Chem. Commun. 1995, 2081. 2.a). R. D. Chambers and T. Chivers. J. Chem. Soc. 1965, 3933. b). D. J. Parks, R. E. H. Spence and W. E. Piers. Angew. Chem. 1995, 107, 895. c). R. E. H. Spence, D. J. Parks, W. E. Piers, M.-A. MacDonald, M. J. Zawarotko and S. J. Rettig. Angew. Chem. 1995, 107, 1337. 3. S. J. Lancaster, M. Thornton-Pett, D. M. Dowson and M. Bochmann. Organometallics 1998, 17, 3829.
References:
Bayer Aktiengesellschaft US6657027, 2003, B2 Location in patent:Page column 15
344-04-7
369 suppliers
$9.00/5g

2720-03-8
10 suppliers
inquiry