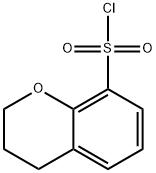
Chroman-8-sulfonyl chloride , 97% synthesis
- Product Name:Chroman-8-sulfonyl chloride , 97%
- CAS Number:1048970-15-5
- Molecular formula:C9H9ClO3S
- Molecular Weight:232.68
3722-78-9
50 suppliers
$90.00/100mg

1048970-15-5
7 suppliers
inquiry
Yield:1048970-15-5 30%
Reaction Conditions:
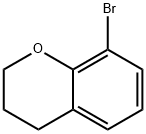
Stage #1: 8-bromo-3,4-dihydro-2H-1-benzopyranwith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;Inert atmosphere;
Stage #2: with sulfur dioxide in tetrahydrofuran;hexane at -78 - 20; for 0.05 h;
Stage #3: with N-chloro-succinimide in dichloromethane at 20; for 1 h;
Steps:
Method O. Chromane-8-sulfonyl chloride (18).
8-Bromochromane (17) was prepared from commercial 2,6-dibromophenol according to the literature procedure [Kerrigan, F.; Martin, C; Thomas, G.H. Tetrah. Lett. 1998, 39, 2219]. To 8-bromochromane (17) (0.1 8 g, 0.84 mmol) in dry tetrahydrofuran (2 mL) at -78° C under argon 2.5 M n-BuLi in hexanes (0.34 ml, 0.85 mmol) was added slowly. The reaction was stirred at this temperature for 30 min and then S02 (g) was bubbled through the solution for 3 minutes. The reaction was stirred at -78° C then warmed to room temperature and the solvent was evaporated. The residue was dissolved in 2 ml of DCM and NCS (0.1 12 g, 0.84 mmol) was added at r.t. , the reaction mixture stirred for 1 h, then diluted with 15 mL DCM, washed with water, brine, dried over sodium sulfate and the solvent was removed under vacuum. The residue was purified by column chromatography on silica gel (eluent petroleum ether-ethyl acetate, 3: 1 ) to give 0.054 g (30%) of compound 18. Compound was unstable under GCMS and LCMS conditions. NMR (CDCI3) δ: 7.78-7.75 (m, 1H), 7.39-7.36 (m, 1H), 6.95 (t, J=7.8 Hz, 1 H), 4.46 (t, J=5.4 Hz, 2H), 2.88 (t, J=6.5 Hz, 2H), 2.16-2.09 (m, 2H).
References:
WO2016/129983,2016,A1 Location in patent:Page/Page column 34