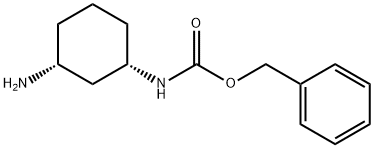
cis-benzyl3-aMinocyclohexylcarbaMate synthesis
- Product Name:cis-benzyl3-aMinocyclohexylcarbaMate
- CAS Number:1259278-12-0
- Molecular formula:C14H20N2O2
- Molecular Weight:248.32
![Carbamic acid, N-[(1S,3R)-3-(aminocarbonyl)cyclohexyl]-, phenylmethyl ester](/CAS/20200611/GIF/1259278-11-9.gif)
1259278-11-9
0 suppliers
inquiry

1259278-12-0
18 suppliers
inquiry
Yield:1259278-12-0 75%
Reaction Conditions:
with bis-[(trifluoroacetoxy)iodo]benzene in water;acetonitrile at 20;
Steps:
Formation of benzyl N-[(1S,3R)-3-aminocyclohexyl]carbamate (18e)
Formation of benzyl N-[(1S,3R)-3-aminocyclohexyl]carbamate (18e) (2018) Benzyl N-[(1S,3R)-3-carbamoylcyclohexyl]carbamate, 18d, (9.1 g, 32.9 mmol) was suspended in a mixture of acetonitrile (100 mL) and water (100 mL) and treated with bis(trifluoroacetoxy)iodobenzene (15.5 g, 36.1 mmol). The suspension was allowed to stir at room temperature overnight and was then quenched with 1N HCl (100 mL). After evaporation of the acetonitrile, the acidic aqueous solution was washed with EtOAc (2×150 mL). The pH was adjusted to basic by addition of solid KOH and the resulting emulsion was extracted with EtOAc (3×200 mL). The combined organic layers were dried over Na2SO4 and concentrated in vacuo to provide product 18e (6.2 g, 75% yield). (2019) 1H NMR (300 MHz, CDCl3) δ 7.31-7.45 (m, 5H), 5.11 (s, 2H), 4.90 (br. s., 1H), 3.58 (br. s., 1H), 2.72-2.97 (m, 1H), 2.14 (d, J=11.90 Hz, 1H), 1.87-2.02 (m, 1H), 1.73-1.87 (m, 2H), 1.21-1.46 (m, 1H), 0.89-1.18 (m, 3H).
References:
US9345708,2016,B2 Location in patent:Page/Page column 300; 301

227783-08-6
29 suppliers
$195.00/100mg

1259278-12-0
18 suppliers
inquiry
![Cyclohexanecarboxylic acid, 3-[[(phenylmethoxy)carbonyl]amino]-, ethyl ester, (1R,3S)-](/CAS/20200611/GIF/1259278-09-5.gif)
1259278-09-5
0 suppliers
inquiry

1259278-12-0
18 suppliers
inquiry

100-51-6
1385 suppliers
$5.00/100g

1259278-12-0
18 suppliers
inquiry