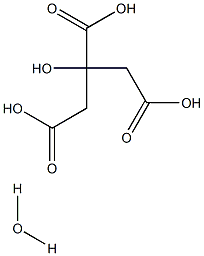
Citric acid monohydrate synthesis
- Product Name:Citric acid monohydrate
- CAS Number:5949-29-1
- Molecular formula:C6H10O8
- Molecular Weight:210.1388
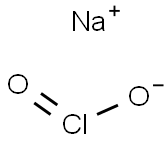
7758-19-2
294 suppliers
$37.79/250ML

5949-29-1
737 suppliers
$10.00/25g
Yield:-
Reaction Conditions:
with sodium hydroxide
Steps:
Sample 14
Sample 14
An aqueous composition was prepared as follows. 1 g (4.8 mmol) of citric acid monohydrate (obtained from Jungbunzlauer) was added to a beaker and neutralized to a pH of 7.4 by addition of 1.2 g (15 mmol) of the 50% sodium hydroxide solution.
Next, 9 g (24.88 mmol) of the 25% sodium chlorite solution, 90 ml of deionized water, and 1 g (6.02 mmol) of the potassium iodide were added to the beaker, and the contents of the beaker were stirred in order to produce the aqueous composition.
The pH of the contents of the beaker was 11.7 while stirring.
At 120 days after the date of preparation of the composition, the pH of the composition was 9.1.
References:
US2018/179058,2018,A1