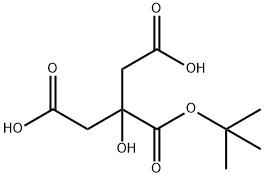
Citric Acid tert-Butyl Ester synthesis
- Product Name:Citric Acid tert-Butyl Ester
- CAS Number:114340-52-2
- Molecular formula:C10H16O7
- Molecular Weight:248.23
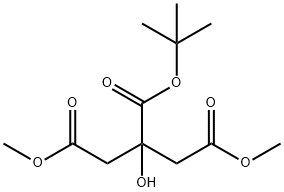
114340-51-1
16 suppliers
inquiry

114340-52-2
20 suppliers
$67.00/100mg
Yield:114340-52-2 86%
Reaction Conditions:
with sodium hydrogencarbonate in methanol;water at 0 - 20; for 5.25 h;
Steps:
A2 Molecule 4: Product Obtained by Saponification of Molecule 3
Molecule 4: Product Obtained by Saponification of Molecule 3 (0333) To a solution of molecule 3 (3.35 g, 12.13 mmol) in methanol (120 mL) at 0° C., a 2N aqueous solution of soda (18.2 mL), cooled beforehand to 0° C., is added. The medium is stirred at 0° C. for 15 min, then at ambient temperature for 5 h. After evaporation of the organic phase under a vacuum, the medium is diluted with water (50 mL), and the aqueous phase is washed with ethyl acetate (100 mL), then acidified with a 10% aqueous solution of HCl and extracted with ethyl acetate (2×150 mL). The organic phase is washed with water (100 mL), a saturated aqueous solution of NaCl (150 mL), then dried over Na2SO4, filtered and evaporated under a vacuum, to yield a white solid. The aqueous phase is extracted again with ethyl acetate (2×150 mL), dried over Na2SO4, filtered and evaporated under a vacuum to yield a second portion of the desired product. (0334) Yield: 15.6 g (86%) (0335) 1H NMR (CD3OD, ppm): 1.46 (9H); 2.71 (2H); 2.86 (2H). (0336) LC/MS (ESI): 247.1; (calculated ([M-H]-): 247.3).
References:
US2017/136097,2017,A1 Location in patent:Paragraph 0333; 0334; 0335; 0336

53798-96-2
14 suppliers
$55.00/100mg

114340-52-2
20 suppliers
$67.00/100mg
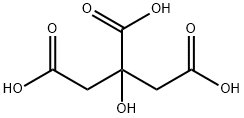
77-92-9
1626 suppliers
$5.00/10g

114340-52-2
20 suppliers
$67.00/100mg