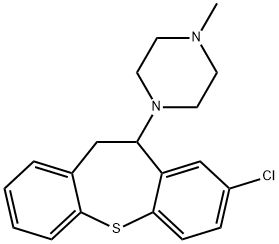
Clorotepine synthesis
- Product Name:Clorotepine
- CAS Number:13448-22-1
- Molecular formula:C19H21ClN2S
- Molecular Weight:344.9
![Piperazine, 1-(8-chlorodibenzo[b,f]thiepin-10-yl)-4-methyl-](/CAS/20210305/GIF/22431-21-6.gif)
22431-21-6
0 suppliers
inquiry

13448-22-1
7 suppliers
inquiry
Yield:13448-22-1 143 mg
Reaction Conditions:
with sodium tetrahydroborate in acetic acid at 20;Inert atmosphere;
Steps:
4.2.31 1-(10,11-Dihydrodibenzo[b,f]thiepin-10-yl)-4-methylpiperazine (41a)
General procedure: Under nitrogen, 38a (132mg, 0.58mmol, 1.0equiv.) was dissolved in 5.8mL of toluene. 1-Methylpiperazine (39a) (0.31mL, 2.86mmol, 4.9equiv.) was added, then Ti(i-OPr)4 (0.13mL, 0.44mmol, 0.75equiv.) and the mixture was refluxed for 20h, then cooled to room temperature. Ice and water were carefully added, and the resulting aqueous solution was extracted with EtOAc (5×). The organic phases were united, washed with brine, dried over Na2SO4, filtered and the solvent was removed under reduced pressure. Thus was obtained 200mg (100% plus impurities) of the intermediate enamine 40a as a yellow oil; the crude was used in the next step without further purification. (0107) The crude intermediate 40a was dissolved in 4.1mL of glacial acetic acid and NaBH4 (329mg, 8.70mmol, 15equiv.) was added in portions. The mixture was stirred at room temperature overnight, then EtOAc was added. The pH of the mixture was adjusted to 6-7 using first NaOH pellets and then NaHCO3, and the resulting aqueous solution was extracted with EtOAc (3×). The organic phases were united, washed with brine, dried over Na2SO4, filtered and the solvent was removed under reduced pressure. The crude residue was purified by flash chromatography (eluent DCM/MeOH=95/5 to 9/1), yielding 99mg (55%) of 1-(10,11-dihydrodibenzo[b,f]thiepin-10-yl)-4-methylpiperazine (41a) as an orange solid.
References:
Boudhar, Aicha;Ng, Xiao Wei;Loh, Chiew Yee;Chia, Wan Ni;Tan, Zhi Ming;Nosten, Francois;Dymock, Brian W.;Tan, Kevin S.W. [European Journal of Medicinal Chemistry,2016,vol. 119,p. 231 - 249]
18698-96-9
235 suppliers
$5.00/250mg

13448-22-1
7 suppliers
inquiry

106-54-7
244 suppliers
$10.00/5g

13448-22-1
7 suppliers
inquiry
![Benzeneacetic acid, 2-[(4-chlorophenyl)thio]-](/CAS/20150408/GIF/13459-62-6.gif)
13459-62-6
36 suppliers
inquiry

13448-22-1
7 suppliers
inquiry

![8-Chlorodibenzo[b,f]thiepin-10(11H)-one](/CAS/20150408/GIF/1469-28-9.gif)