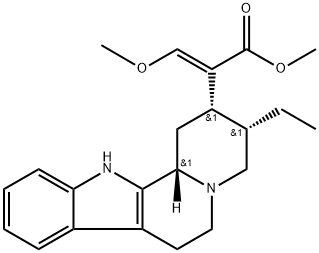
corynantheidine synthesis
- Product Name:corynantheidine
- CAS Number:23407-35-4
- Molecular formula:C22H28N2O3
- Molecular Weight:368.48
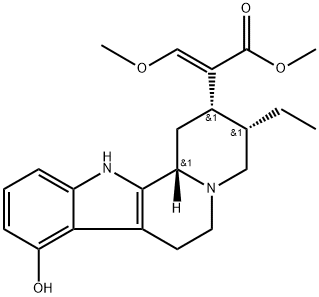
425623-55-8
2 suppliers
inquiry

23407-35-4
6 suppliers
inquiry
Yield:23407-35-4 1.17 g
Reaction Conditions:
Stage #1: (-)-9-Hydroxycorynantheidinewith dmap;N,N-phenylbistrifluoromethane-sulfonimide;triethylamine in dichloromethane at 20; for 2 h;Inert atmosphere;
Stage #2: with 5%-palladium/activated carbon;hydrogen in methanol at 20; under 760.051 Torr; for 2 h;
Steps:
12 Corynantheidine.
To a solution of 9-hydroxycorynantheidine (2.00 g, 5.20 mmol) in anhydrous CH2CI2 (139 mL) under argon at room temperature was added 4 -( dimethylamino ) pyridine (127 mg, 1.04 mmol), Et3N (1.45 mL, 1.05 g, 10.40 mmol), and N-phenyl- bis ( trifluoromethanesulfonimide ) (2.42 g, 6.76 mmol), and the resulting brown solution was left to stir at room temperature. After 2 h, the reaction mixture was concentrated in vacuo to give a sticky dark-brown glass (5.27 g) . This material was purified directly by column chromatography (8:2 hexanes : EtOAc, 3 column volumes 7:3 hexanes : EtOAc, 2 column volumes 1:1 hexanes : EtOAc, 1 column volume) to give the triflate intermediate containing impurities as a very pale-yellow foam (2.49 g) . A quantity (2.45 g) of this material was combined with 5% Pd on carbon (2.45 g) , MeOH (47.5 mL) was added, and the mixture was stirred at room temperature under 1 atm for 2 h. The mixture was then filtered through celite, the filter cake was washed with MeOH (3 x 50 mL) , and the combined filtrates concentrated in vacuo to give the crude product as a- pale-yellow foam (2.12 g) . This material was purified by column chromatography (8:2 hexanes : EtOAc + 2% Et3N, 3 column volumes - 7:3 hexanes : EtOAc + 2% Et3N, 3 column volumes) to give pure corynantheidine as an amorphous off-white solid (1.17 g, 62% over 2 steps) .1H NMR (500 MHz, CDC13) d 7.76 (br s, 1H), 7.47 (d, J= 7.6 Hz, 1H), 7.44 (s, 1H), 7.30 (d, J = 7.9 Hz, 1H), 7.15 - 7.04 (m, 2H) , 3.73 (s, 3H), 3.72 (s, 3H), 3.19 (dd, J = 11.4, 2.4 Hz, 1H), 3.09 - 2.93 (m, 4H) , 2.74 - 2.66 (m, 1H) , 2.62 - 2.46 (m, 3H), 1.88 - 1.73 (m, 2H), 1.68 - 1.60 (m, 1H) , 1.28 - 1.16 (m, 1H) , 0.88 (t, J = 7.4 Hz, 3H ) ;13C NMR (126 MHz, CDC13) d 169.4, 160.7, 136.0, 135.7, 127.7, 121.3, 119.4, 118.2, 111.6, 110.8, 108.2, 61.7, 61.4, 57.9, 53.6, 51.5, 40.8, 40.1, 30.0, 22.0, 19.2, 13.0.
References:
WO2020/160280,2020,A1 Location in patent:Page/Page column 93-95
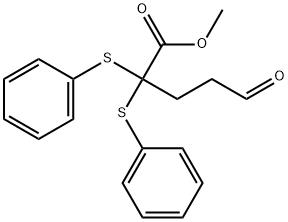
84406-10-0
0 suppliers
inquiry

23407-35-4
6 suppliers
inquiry
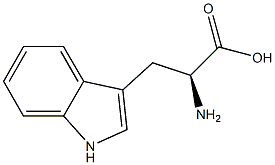
765229-74-1
4 suppliers
inquiry

23407-35-4
6 suppliers
inquiry
141595-98-4
21 suppliers
$216.00/1g

23407-35-4
6 suppliers
inquiry

153-94-6
614 suppliers
$5.00/5g

23407-35-4
6 suppliers
inquiry