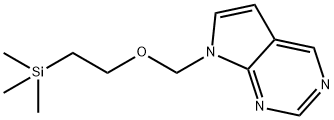
CP-1036 synthesis
- Product Name:CP-1036
- CAS Number:1001070-45-6
- Molecular formula:C12H19N3OSi
- Molecular Weight:249.38
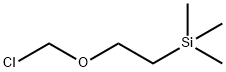
76513-69-4
354 suppliers
$6.00/1g

1001070-45-6
10 suppliers
inquiry
Yield:1001070-45-6 35%
Reaction Conditions:
Stage #1: 7H-pyrrolo[2,3-d]pyrimidinewith sodium hydride in tetrahydrofuran at 0 - 20;
Stage #2: (2-trimethylethylsilylethoxy)methyl chloride in tetrahydrofuran at 20; for 2 h;
Steps:
3
7H-Pyrrolo[2,3-d]pyrimidine (1.16 g, 9.74 mmol), was dissolved in 100 ml anhydrous THF under N2. At 0° C., a 60% dispersion of NaH 0.51 g in mineral oil was added. The mixture was stirred at ambient temperature and 2.93 ml (9.8 mmol) (2-chloromethoxy-ethyl)-trimethyl-silane dissolved in 15 ml anhydrous THF was added. The reaction mixture was stirred at room temperature for 2 hour and subsequently concentrated in vacuo. Ethyl acetate was added to the mixture and the organic layer was washed three times with a saturated NaHCO3 solution, dried (Na2SO4), filtered and concentrated. The resulting residue was purified by flash chromatography (diethyl ether) to afford 7-(2-trimethylsilanyl-ethoxymethyl-7H-pyrrolo[2,3-d]pyrimidine (compound 91), (amorphous, 0.84 g, 35%). 1H-NMR (400 MHz, CDCl3): δ 9.1 (bs, 1H), 9.0 (bs, 1H), 7.43 (bd, J=4 Hz, 1H), 6.68 (bd, J=4 Hz, 1H), 5.73 (s, 1H), 3.6 (t, J=8 Hz, 2H), 0.97 (t, J=8 Hz, 2H), 0.1 (s, 9H).
References:
US2008/9514,2008,A1 Location in patent:Page/Page column 27