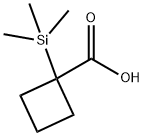
Cyclobutanecarboxylic acid, 1-(trimethylsilyl)- synthesis
- Product Name:Cyclobutanecarboxylic acid, 1-(trimethylsilyl)-
- CAS Number:97592-20-6
- Molecular formula:C8H16O2Si
- Molecular Weight:172.3
Yield:97592-20-6 23%
Reaction Conditions:
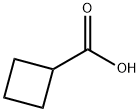
Stage #1:Cyclobutanecarboxylic acid with lithium diisopropyl amide in tetrahydrofuran at 20; for 4 h;Inert atmosphere;Cooling with ice;
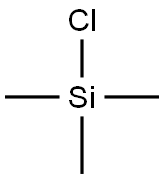
Stage #2:chloro-trimethyl-silane with N,N,N,N,N,N-hexamethylphosphoric triamide in tetrahydrofuran at -78 - -60; for 16.5 h;Inert atmosphere;
Steps:
1 1-(Trimethylsilyl)cyclobutanecarboxylic acid
To 200 ml of dehydrated THF, 214 ml (428 mmol) of 2 M lithium diisopropylamide/THF solution was added in an argon atmosphere, and then, 10.1 ml (107 mmol) of cyclobutanecarboxylic acid was added dropwise with stirring under cooling in ice water and stirred for 4 hours while the temperature was raised to room temperature for a while.
Subsequently, 20 ml (116 mmol) of hexamethylphosphoric acid triamide was added, and 51 ml (490 mmol) of chlorotrimethylsilane was added dropwise with stirring while the internal temperature was kept at -60°C or lower under cooling in a dry ice/acetone coolant, and then stirred at -78°C for 16.5 hours.
After the completion of the reaction, 67 ml of methanol was added to the reaction solution, and 134 ml of cold water was added after the temperature was raised to 0°C.
The pH was adjusted to 2.1 by adding 2 N hydrochloric acid, 268 ml of diethyl ether was added thereto and then the aqueous layer and the organic layer were separated, and the organic layer was washed with 268 ml of a saturated aqueous solution of sodium chloride.
The organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure.
50 ml of a 2 N aqueous sodium hydroxide solution and 267 ml of n-hexane were added to the obtained concentration residue and then the aqueous layer and the organic layer were separated.
Subsequently, the aqueous layer was adjusted to pH 2.7 by adding 1 N hydrochloric acid, and 267 ml of ethyl acetate was added to this solution and then the aqueous layer and the organic layer were separated.
The obtained organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure.
n-Hexane was added to the obtained concentration residue and cooled in an ice water bath.
The resulting solid was filtered, washed by sousing with cooled n-hexane, and then dried under reduced pressure to obtain 6.24 g of the title compound (yield: 34%) as a white solid.
References:
Ube Industries, Ltd.;AGA, Yasuhiro;USHIYAMA, Shigeru;IWASE, Noriaki;KONO, Shigeyuki;SUNAMOTO, Hidetoshi;MATSUSHITA, Takashi;OGI, Sayaka;TANAKA, Masayuki;MATOYAMA, Masaaki;UMEZAKI, Satoshi;SHIRAISHI, Yusuke;ONUMA, Kazuhiro;KOJIMA, Masahiro;NISHIYAMA, Hayato;KIMURA, Tomio EP3214086, 2017, A1 Location in patent:Paragraph 0656-0658