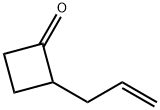
Cyclobutanone, 2-(2-propen-1-yl)- synthesis
- Product Name:Cyclobutanone, 2-(2-propen-1-yl)-
- CAS Number:41780-92-1
- Molecular formula:C7H10O
- Molecular Weight:110.15
Yield:41780-92-1 90%
Reaction Conditions:
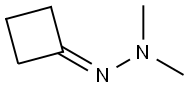
Stage #1: N-cyclobutylidene-(N',N'-dimethylamino)aminewith n-butyllithium in diethyl ether;hexane at 0 - 10; for 1.33333 h;Inert atmosphere;
Stage #2: allyl bromide in diethyl ether;hexane at 20;
Steps:
90.2 STEP 2: (S)-2-ALLYLCYCLOBUTANONE AND (R)-2-ALLYLCYCLOBUTANONE
To a stirred, Ca. -10 °C solution of 2-cyciohutyhdene-1,i-dimethyihydrazine (12,0 g, 107 mmoi) in diethyl ether (206 mL) under a nitrogenatmosphere was added n-butyliithium (nominally 2.5 M solution in hexanes; 42.8mL. 107 mmol) dropwise via syringe over 20 minutes. The reaction mixture was stirred at -10 °C for one hour. After this time. ailvi bromide (9,26 mL, 107 mmol) was added, and the mixture was allowed to warm to room temperature overnight. After this time, the mixture was acidified with aqueous HC1 (1 M, 215 mL) andstirred at rt for 40 minutes. The separated aqueous layer was then extracted with diethyl ether, and the combined organic extracts were washed with brine, dried over MgSO4, filtered and concentrated in vacuo (pressure: 100 mm of Hg) to give 2-alivicyclohutanone (10.55 g, 96.0 mmol. 90 % yield).
References:
WO2017/147410,2017,A1 Location in patent:Page/Page column 312; 313