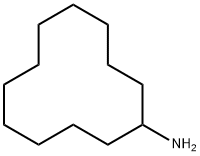
CYCLODODECYLAMINE synthesis
- Product Name:CYCLODODECYLAMINE
- CAS Number:1502-03-0
- Molecular formula:C12H25N
- Molecular Weight:183.33
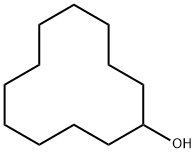
1724-39-6
122 suppliers
$7.00/5g

1502-03-0
83 suppliers
$20.00/250mg
Yield: 1.427 g
Reaction Conditions:
with carbonylchlorohydrido(4,5-bis((diisopropylphosphino)methyl)acridine)ruthenium(II);ammonia in tert-Amyl alcohol at 20 - 170; under 11251.1 - 15001.5 Torr; for 48 h;Inert atmosphere;Autoclave;
Steps:
4 Example 4 Direct Single-Stage Amination of Cyclododecanol by Means of Ammonia Over a Ruthenium-Pincer Complex (Vliq/Vgas=0.3, According to the Invention)
Under an argon atmosphere, 1.843 g (10 mmol) of cyclododecanol, 0.030 g (0.05 mmol) of carbonylchlorohydrido[4,5-(di-i-propylphosphinomethylacridino)ruthenium(II)] as catalyst and 25 ml of 2-methyl-2-butanol as solvent are placed in the glass liner of a 100 ml Hastelloy autoclave.
The autoclave is closed, pressurized with 20 bar of argon and vented three times and again pressurized with 15 bar of argon. 2 g (117.6 mmol) of liquid ammonia are then introduced into the autoclave (overall Vliq/Vgas=0.3).
The reaction mixture is stirred for 10 minutes at room temperature (600 rpm), subsequently heated while stirring to an internal temperature of 170° C. and maintained at this temperature for 48 hours.
After cooling to room temperature, careful depressurization of the mixture and pressurization with 20 bar of argon three times with subsequent venting, the autoclave is opened, the reaction mixture filtered through kieselguhr and the filtrate is evaporated under reduced pressure on a rotary evaporator to remove the solvent.
The crude product obtained is purified by bulb tube distillation under reduced pressure.
This gives 1.427 g of cyclododecylamine (yield: 78% of theory; boiling range: 175-180° C. air bath temperature at 6 mbar).
References:
Evonik Degussa GmbH;Klasovsky, Florian;Pfeffer, Jan Christoph;Tacke, Thomas;Haas, Thomas;Martin, Andreas;Deutsch, Jens;Koeckritz, Angela US2013/165672, 2013, A1 Location in patent:Paragraph 0078

1724-39-6
122 suppliers
$7.00/5g

1502-03-0
83 suppliers
$20.00/250mg
830-13-7
171 suppliers
$16.00/25g

830-13-7
171 suppliers
$16.00/25g

1502-03-0
83 suppliers
$20.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1502-03-0
83 suppliers
$20.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1502-03-0
83 suppliers
$20.00/250mg