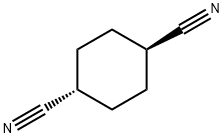
CYCLOHEXANE-1,4-DICARBONITRILE synthesis
- Product Name:CYCLOHEXANE-1,4-DICARBONITRILE
- CAS Number:6550-85-2
- Molecular formula:C8H10N2
- Molecular Weight:134.18
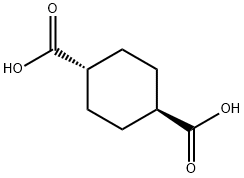
619-82-9
220 suppliers
$5.00/5g

6550-85-2
8 suppliers
inquiry
Yield:6550-85-2 90.2%
Reaction Conditions:
with 1,3-dimethyl-2-imidazolidinone;ammonia;tin(II) oxide at 170 - 280; for 14 h;
Steps:
1
In a flask equipped with a stirrer, a thermometer, a gas introducing tube, a gas exhaust tube, and a gas cooling apparatus, 100 parts by mass of 1,4-cyclohexane dicarboxylic acid obtained in the nuclear hydrodesulfurization step, 100 parts by mass of N, N'-dimethylimidazolidinone , And 1.26 parts by mass of tin (II) oxide were charged and heated to 170 . Thereafter, while stirring at 500 rpm, ammonia gas was introduced at a rate of 0.58 mol / hr (vs.1,4-cyclohexane dicarboxylic acid), the temperature was raised to 280 DEG C, and the reaction was carried out at this temperature for 14 hours. After completion of the reaction, the reaction mixture was cooled to 150 ° C and filtered at the time of heating (at the time of heating) to remove solids. The filtrate was analyzed to find that the conversion of 1,4-cyclohexane dicarboxylic acid was 100% and the yield of 1,4-dicyanocyclohexane was 90.2%The lance phase ratio was 52 mol%, and the concentration of N, N'-dimethylimidazolidinone was 6.9 mass%.
References:
KR101598769,2016,B1 Location in patent:Paragraph 0656; 0657

10534-13-1
15 suppliers
$156.00/1g

6550-85-2
8 suppliers
inquiry
54657-09-9
7 suppliers
inquiry

6550-85-2
8 suppliers
inquiry
100-21-0
551 suppliers
$6.00/25g

6550-85-2
8 suppliers
inquiry
20101-86-4
4 suppliers
inquiry

6550-85-2
8 suppliers
inquiry