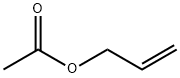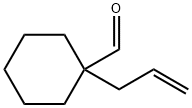
Cyclohexanecarboxaldehyde, 1-(2-propen-1-yl)- synthesis
- Product Name:Cyclohexanecarboxaldehyde, 1-(2-propen-1-yl)-
- CAS Number:29517-58-6
- Molecular formula:C10H16O
- Molecular Weight:152.23
Yield:29517-58-6 78%
Reaction Conditions:
toluene-4-sulfonic acid in benzene at 130 - 180; for 15 h;
Steps:
1
A mixture of cyclohexanecarboxaldehyde ( (10OmL, 0.825 mol), allyl alcohol (6OmL, 98% purity, 0.882 mol), benzene (15 mL), and /?-toluenesulfonic acid monohydrate (0.120 g, 0.63 mmol) was heated from 130 0C to 180 0C in an oil bath, under a Vigreaux distillation column, topped by a Dean-Stark trap. After 15 hour no more water was added over the 15 mL collected in the trap, and the reaction was judged completed. Subsequent distillation of the reaction crude gave 98 g (78% yield) of 1- allyl-cyclohexanecarboxaldehyde as a colourless liquid (b.p. 69-71 ^QSnTm Hg). 1H-NMR (200 MHz, CDCl3): δ 1.22-1.65 (1OH, m, -(CHz)5-); 2.17 (2η, m, -CH2- CH=); 4.96-5.07 (2H, m, CH=CH2); 5.55-5.76 (1η, m, CH=CH2); 9.44 (IH, s, CHO). 13C-NMR (200 MHz, CDCl3): δ 22.41 (CH2); 25.61 (CH2); 30.73 (CH2); 40.72 (CH2); 49.56 (C"); 118.21 (CH2=); 132.64 (CH=); 206.70 (CHO). IR (film): 1730 cm"1 (C=O); 2861 cm 1 (CHO). MS (EI 70 eV): m/z 152 (3%, M+); 137 (5); 134 (6); 123 (18); 110 (37); 108 (8); 97
References:
WO2008/4115,2008,A1 Location in patent:Page/Page column 11-12

107-18-6
2 suppliers
$24.60/100ml
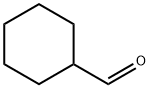
2043-61-0
325 suppliers
$6.00/5g

29517-58-6
2 suppliers
inquiry
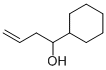
69036-26-6
4 suppliers
inquiry
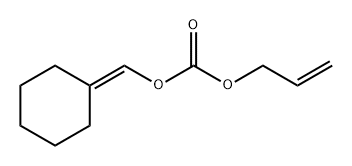
86950-88-1
0 suppliers
inquiry

29517-58-6
2 suppliers
inquiry