
Cyclohexanone,3-methyl-, (3S)- synthesis
- Product Name:Cyclohexanone,3-methyl-, (3S)-
- CAS Number:24965-87-5
- Molecular formula:C7H12O
- Molecular Weight:112.1696
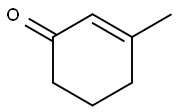
1193-18-6
167 suppliers
$6.00/1g

24965-87-5
4 suppliers
inquiry
Yield:24965-87-5 99%
Reaction Conditions:
Stage #1: 3-methylcyclohexen-2-one;C9H19NO2*C50H57O4P in dibutyl ether at 60; for 0.0333333 - 0.0833333 h;
Stage #2: with C15H23NO4 in dibutyl ether at 60; for 48 h;Product distribution / selectivity;
Steps:
12.d
Asymmetric Transfer Hydrogenation of α,β-Unsaturated Ketones (New Chapter)The process can also be applied to α,β-unsaturated ketones. Especially chiral phosphate salts of primary amino acid esters have been found to be high performance and highly enantioselective catalysts.For instance, the salt 7a, in the presence of the Hantzsch ester 8, catalyzes the highly enantioselective transfer hydrogenation of various α,β-unsaturated ketones (9) (Scheme 12).Further catalytic salts for the enantioselective transfer hydrogenation are shown in Schemes 13-15. Scheme 12. Inventive highly enantioselective transfer hydrogenations. R1 R2 R3 Yield ee (a)-(CH2)2- Me 78% 98% (b)-(CH2)2- Et 71% 97% (c)-(CH2)2- CH2CH2Ph 68% 96% (d)-(CH2)3- Me 99% 94% (e)-(CH2)3- Et 98% 96% (f)-(CH2)3- i-Bu 89% 95% (g)-(CH2)3- i-Pr 94% 98% (h)-(CH2)3- CH2CH2Ph 99% 96% (i)-(CH2)3- Ph 99% 84% (j)-(CH2)4- Me >99% 97% (k) Me MeCO2Et >99% 83% (l) Me Me Ph 81% 69% Asymmetric Transfer HydrogenationThe ketone (9a-l) (1 eq) and catalyst (7a-s) (0.1 eq for 9a-c, or 0.05 eq for 9d-1) were initially charged in Bu2O (0.33 ml/mmol), and the mixture was stirred at 60° C. for 2-5 min. Thereafter, Hantzsch ester (8) (1.2 eq) was added and the mixture was stirred for a further 48 hours. The reaction mixture was supplemented with sodium hydroxide solution (2N, 40 ml/mmol) and extracted with diethyl ether (3×40 ml/mmol). The combined organic phases were dried over magnesium sulfate and concentrated on a rotary evaporator. Column chromatography (pentane/diethyl ether) gave the products in the yields and enantiomeric excesses reported.For the volatile saturated ketones, and also for the examples shown in Schemes 13-15, a sample was taken and the conversion was determined by means of GC.
References:
US2009/30216,2009,A1 Location in patent:Page/Page column 7; 8

625-96-7
1 suppliers
inquiry

24965-87-5
4 suppliers
inquiry
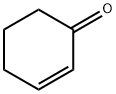
930-68-7
254 suppliers
$9.00/1g

544-97-8
128 suppliers
$150.00/5g

24965-87-5
4 suppliers
inquiry

13368-65-5
36 suppliers
$114.15/5G