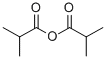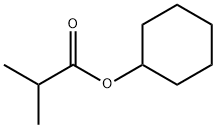
CYCLOHEXYL ISOBUTYRATE synthesis
- Product Name:CYCLOHEXYL ISOBUTYRATE
- CAS Number:1129-47-1
- Molecular formula:C10H18O2
- Molecular Weight:170.25
Yield:1129-47-1 99.5%
Reaction Conditions:
4-N,N-dimethylaminopyridinium saccharinate at 25; for 2 h;Product distribution / selectivity;
Steps:
3
Various secondary alcohols were esterified according to the following scheme by using the method of Example 2, and the results were listed in Table 1. TABLE 1 Temp. Time No. Sec. Alcohol R (° C.) (hr.) Yield (%) 1 1-cylco- Me 25 2.5 99.5, 99.1, 99.7, 96.6, hexanol 97.4, 96.8, 97.6, 93.5, 92.6, 86.6 2 1-cylco- iPr 25 2 >99.5, >99.5, >99.5, >99.5, hexanol >99.5, >99.5, 99, 99, 98, 98 3 1-phenyl- Me 25 2 >99.5, >99.5, >99.5, >99.5, ethanol >99.5, >99.5, >99.5, >99.5, >99.5, >99.5 4 1-phenyl- iPr 25 2 >99.5, >99.5, >99.5, >99.5, ethanol >99.5, >99.5, >99.5, >99.5, >99.5, >99.5 5 menthol Me 25 8-12 97, 96, 96, 96, 95, 92, 95, 98, 92, 87 6 menthol iPr 25 8-12 98, 98, 96, 96, 97, 95, 97, 99, 95, 92 7 1-cyclo- Me 25 8 99, 98, 99, 97, 97, 97, 96, dodecanol 97, 97 8 1-cyclo- iPr 25 8 95, 95, 95, 95, 94, 94, 95, dodecanol 95 9 4-nitro-phenol Me 25 2-4 >99.5, >99.5, >99.5, >97, >99.5, >99.5, 97.5, 96.4, 96, 96 10 4-nitro-phenol iPr 25 2-4 99.5, 99.5, 99.5, 99.5, 99.5, 99.5, 99.5, 99.5, 99.5, 99.5 From the results of No. 1 and No. 2 in Table 1, it can be known that the catalyst of Example 1 can catalyze the esterification of secondary alcohols. Since it is more difficult for a secondary alcohol to be esterified than a primary alcohol, the catalyst of Example 1 can surely catalyze the esterification of a primary alcohol. In addition, the catalyst of Example 1 can catalyze the reaction of an alcohol not only with acetic anhydride (see No. 1), but also with isobutyric anhydride, which has higher steric hindrance (see No. 2). Furthermore, it can be known from the yields that the effect of the catalyst did not decrease even after the catalyst was reused many times. For example, as shown by the results of No. 2 in Table 1, the yield was still as high as 98% after the catalyst was reused 10 times.From the results of No. 3 to 10, it can be known that the catalyst of Example 1 can also catalyze the reaction of secondary alcohols other than 1-cyclohexanol with various acid anhydrides. 1-phenylethanol (Nos. 3 and 4) can be esterified with high yield under substantially the same conditions as those for esterification of 1-cyclohexanol. For menthol (Nos. 5 and 6), longer reaction time (about 8 hours) was needed since menthol had higher steric hindrance than that of 1-cyclohexanol. For 1-cyclododecanol (Nos. 7 and 8), the reaction time is about 8 hours, and high yield was obtained even after the catalyst had been reused 8 times. In addition, for phenols with lower nucleophilicity, such as 4-nitrophenol (Nos. 9 and 10), the catalyst also showed good catalytic effect on acylation thereof since the reaction can be completed in 4 hours and the average yield was still above 98% even after the catalyst was reused 10 times.
References:
US2012/184748,2012,A1 Location in patent:Page/Page column 3