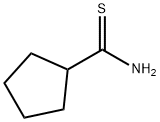
CYCLOPENTANECARBOTHIOAMIDE synthesis
- Product Name:CYCLOPENTANECARBOTHIOAMIDE
- CAS Number:42202-73-3
- Molecular formula:C6H11NS
- Molecular Weight:129.22
3217-94-5
33 suppliers
$29.00/1g

42202-73-3
20 suppliers
inquiry
Yield:42202-73-3 82%
Reaction Conditions:
with Lawessons reagent in tetrahydrofuran;toluene at 70;
Steps:
5
Intermediate 5; Cyc1opentanecarbothioamide; [00138] Ammonia was bubbled through a solution of cyclopentanecarbonyl chloride (5.46 g, 41.1 mmol) in THF (60 mL) for 30 seconds. After 10 min, the ammonium chloride produced was filtered off and the filtrate concentrated. Then added EtOAc and water and separated the layers and re- extracted with a further portion of EtOAc. The combined organics were dried (MgSO4), filtered and concentrated in vacuo to give the amide (4.46 g, 96%) as a clear oil. The
References:
WO2007/117692,2007,A2 Location in patent:Page/Page column 51-52
3400-45-1
358 suppliers
$10.00/5g

42202-73-3
20 suppliers
inquiry

4524-93-0
134 suppliers
$13.43/1gm:

42202-73-3
20 suppliers
inquiry