
Cyclopropyl(2-fluorophenyl)methanol synthesis
- Product Name:Cyclopropyl(2-fluorophenyl)methanol
- CAS Number:844470-89-9
- Molecular formula:C10H11FO
- Molecular Weight:166.19
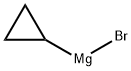
23719-80-4
170 suppliers
$85.20/25ml

446-52-6
425 suppliers
$6.00/25g

844470-89-9
7 suppliers
$504.00/1g
Yield:844470-89-9 62%
Reaction Conditions:
in tetrahydrofuran at 20; for 16 h;Cooling with ice;Inert atmosphere;
Steps:
111.1 Step 1: preparation of cyclopropyl(2-fluorophenyl)methanol
2-fluorobenzaldehyde (1.0 g, 8 mmol) was dissolved in THF (20 mL), and the reaction solution was cooled in an ice bath. With purging three times with nitrogen, cyclopropylmagnesium bromide (32 mL, 16 mmol) was added dropwise. Upon completion of the addition, the reaction was warmed up to room temperature gradually, carried out for 16 h, quenched with 20 mL of saturated ammonium chloride aqueous solution and extracted with ethyl acetate (50 mL * 3). The organic phase was dried over anhydrous sodium sulfate and concentrated to obtain a crude product which was further purified by column chromatography to obtain cyclopropyl(2-fluorophenyl)methanol (800 mg, 62%).
References:
EP3205650,2017,A1 Location in patent:Paragraph 0580; 0581
4333-56-6
377 suppliers
$6.00/5g

446-52-6
425 suppliers
$6.00/25g

844470-89-9
7 suppliers
$504.00/1g