
D-a-Methyl DOPA synthesis
- Product Name:D-a-Methyl DOPA
- CAS Number:2799-15-7
- Molecular formula:C10H13NO4
- Molecular Weight:211.21
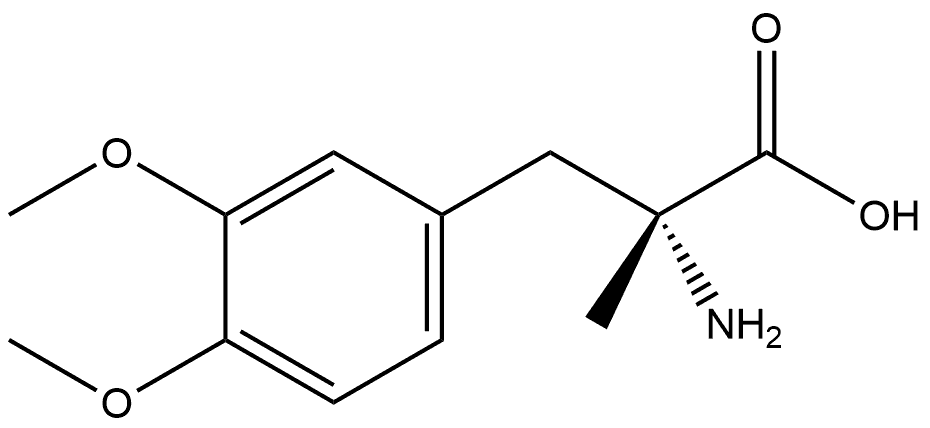
2503-42-6
1 suppliers
inquiry

2799-15-7
28 suppliers
inquiry
Yield:2799-15-7 71.8%
Reaction Conditions:
with hydrogen bromide;ammonia hydrochloride in phenol at 85; for 10 h;
Steps:
1-4 Preparation of D-α-methyldopa (Step (1-d))
Compound 4 with 1 eq. of ammonium chloride (3.0 g, 9.1 mmol) was charged into 20 mL reaction vial, followed by phenol (1.7 g, 18.2 mmol, 2.0 eq) and 48% HBr solution (60 mL, 89.4 g, 530.3 mmol). The mixture was heated at 85±5° C. Samples were pulled for HPLC assay. Results of HPLC analysis indicated that reaction reached completion after 10 h. The mixture was cooled to room temperature and then was concentrated in vacuo to remove HBr solution at a bath temperature 60° C. under reduced pressure. HBr solution (50 mL) was removed during the evaporation. The residue was chased with toluene (100 mL×2). HPLC analysis of distillate and residue indicated that phenol was removed from residue during the evaporation. Water (4 mL) was added to obtain a clear solution. The resulting solution was cooled to 0-5° C. The pH was adjusted with 2.0 N ammonium hydroxide solution. After 2.0 N ammonium hydroxide (25 mL) was added the pH of the mixture was still 1.0. The mixture was concentrated in vacuo to a volume of 12 mL and then cooled to 0-5° C. Ammonium hydroxide solution (28-29%) and conc. HCl solution were used for adjustment of pH to 4.5. Solid precipitated and was collected by filtration, followed by a rinse with D. I. water (4 mL×2). The wet solid (2.1 g) was dried at room temperature under reduced pressure for 4 days to give the product (1.38 g). The isolated yield was 71.8%.
References:
US2022/194893,2022,A1 Location in patent:Paragraph 0014; 0021
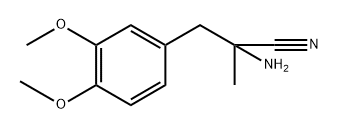
2543-46-6
4 suppliers
inquiry

2799-15-7
28 suppliers
inquiry
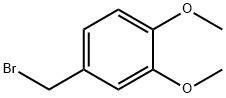
21852-32-4
117 suppliers
$50.00/250mg

2799-15-7
28 suppliers
inquiry