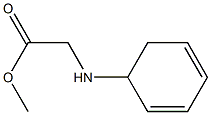
D-dihydrophenylglycine methyl ester synthesis
- Product Name:D-dihydrophenylglycine methyl ester
- CAS Number:104236-44-4
- Molecular formula:C9H13NO2
- Molecular Weight:167.21
Yield:104236-44-4 34.64%
Reaction Conditions:
with sulfuric acid at 60 - 70;Inert atmosphere;Reflux;Large scale;
Steps:
Preparation of methyl (R)-2-amino-2-(cyclohexa-1 ,4-dien-1-yl)acetate solution(DHPGM)
Under nitrogen atmosphere (R)-2-amino-2-(cyclohexa-1 ,4-dien-1-yl)acetic acid(D-dihydrophenylglycine, DHPG; about 1250-1 500 kg) was suspended in methanol, at a ratio of 1 kg/1.1-2.0 L, while cooling down. The suspension was mixed with a mixture of methanol (about 0.7 L/kg DHPG) and 98% sulfuric acid (about 0.4 L/kg DHPG), while keeping temperature below 70°C. Subsequently, the mixture was heated to reflux andmaintained for about 1-3 hours, then cooled concentrated under reduced pressure, until the temperature is less than 60°C. After cooling, methanol (about 0.3-1.5 L/kg DHPG) was added, and the mixture was heated to reflux and then distilled, as described above. This addition-reflux-distillation profile was repeated until a conversion of not less than 97% was reached. The mixture was then cooled. Ammonia (25%) wasdosed until a pH of 1 .7-3.4, maintaining temperature. Water was added (about 1 L/kg DHPG) and the solution was distilled under vacuum to remove methanol. Finally, the mixture comprising methyl (R)-2-amino-2-(cyclohexa-1 ,4-dien-1-yl)acetate (34.64%, pH = 2) was cooled and stored for further use.
References:
WO2017/21390,2017,A1 Location in patent:Page/Page column 9

