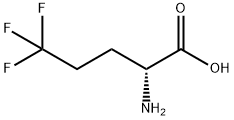
D-Norvaline, 5,5,5-trifluoro- synthesis
- Product Name:D-Norvaline, 5,5,5-trifluoro-
- CAS Number:122565-29-1
- Molecular formula:C5H8F3NO2
- Molecular Weight:171.12
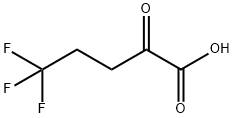
118311-18-5
10 suppliers
inquiry

122565-29-1
20 suppliers
$583.00/1g
Yield:122565-29-1 94%
Reaction Conditions:
with potassium phosphate;pyridoxal 5'-phosphate;sodium formate;rac-Ala-OH;L-lactate dehydrogenase;formate dehydrogenase;R-transaminase AT-103;NAD in water at 30; pH=7.5;Product distribution / selectivity;
Steps:
B.A
The yields were increased by adding auxiliary enzymes to reduce pyruvate to lactate. Lactate dehydrogenase requires NADH as a cofactor. The NADH was regenerated using formate dehydrogenase. A solution containing 5,5,5-trifluoro-2-oxopentanoic acid (100 mg, 0.588 mmoles), D,L-alanine (200 mg, 2.244 mmoles), 0.02 mM pyridoxal phosphate, sodium formate (68 mg, 1 mmole), NAD (3.31 mg, 5 μmoles) L-lactate dehydrogenase cloned from rabbit muscle (Biocatalytics LDH-103, 0.107 mg, 15 units), and formate dehydrogenase (0.5 mL, 15 units cloned from Pichia pastoris and expressed in Escherichia coli) in 0.1 M potassium phosphate buffer, pH 7.5, was incubated with R-transaminase AT-103 from Biocatalytics (5 mg, 44units) or cloned R-transaminase from Bacillus thuringiensis SC16569 (0.5 mL, 10 units) at 30° C. in a total volume of 5 mL in 15 mL tubes. Reaction yields of (R)-5,5,5-trifluoro-2-aminopentanoic acid of 94% and 91% were obtained with AT-103 and BMS transaminases, respectively. Ee was 100% in both cases.
References:
US2009/111858,2009,A1 Location in patent:Page/Page column 16

122489-77-4
0 suppliers
inquiry

122565-29-1
20 suppliers
$583.00/1g
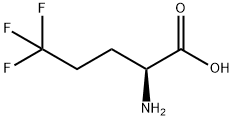
2365-80-2
18 suppliers
$75.00/100mg

122565-29-1
20 suppliers
$583.00/1g