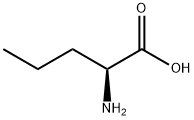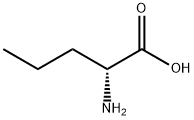
D(-)-Norvaline synthesis
- Product Name:D(-)-Norvaline
- CAS Number:2013-12-9
- Molecular formula:C5H11NO2
- Molecular Weight:117.15
Yield: 88 % ee
Reaction Conditions:
with isopropylamine in aq. buffer; pH=7.0 for 48 h;Enzymatic reaction;Overall yield = 90 %;Time;
Steps:
2.7 Preparative-Scale Deracemization
General procedure: Deracemization of dl-1a in a preparative-scale was carried out in a 25 mL reaction mixture (50 mM Tris buffer, pH 7.0)charged with 0.22 g dl-1a (2.5 mmol), 0.15 g 3 (2.5 mmol),17 mg NAD+,1750 U AlaDH, 40 U ARTAmut and 250 U NOX. Reaction was carried under magnetic stirring at 37 °C.Aliquots (typically 10 μL) of the reaction mixture were takenat predetermined reaction time and mixed with 40 μL of 5 NHCl after 20-fold dilution with water. Quantitative chiral analysis of dl-1a was performed by HPLC for determination of eeD. When eeD of d-1a exceeded 99%, reaction was stopped and then product isolation was performed. The pH of the reaction mixture was adjusted to 1.0 byadding 5 N HCl and then filtered using a glass-fritted filter funnel to remove protein precipitates. The filtrate was loaded on a glass column packed with Dowex 50WX8 cation exchange resin (40 g), followed by washing with 0.1 N HCl(100 mL) and then water (50 mL). Elution was performed by 10% (v/v) aqueous ammonia solution (150 mL) and then the eluate was evaporated at 70 °C and 0.25 bar. The resulting white solid was washed with ethanol (20 mL) and dried overnight at 37 °C.
References:
Han, Sang-Woo;Shin, Jong-Shik [Catalysis Letters,2018,vol. 148,# 12,p. 3678 - 3684]
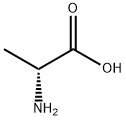
338-69-2
592 suppliers
$5.00/10g
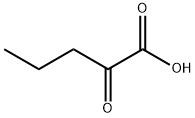
1821-02-9
65 suppliers
$60.00/5mg

2013-12-9
290 suppliers
$5.00/1g
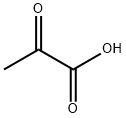
127-17-3
518 suppliers
$5.00/10g
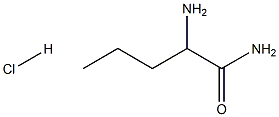
93029-42-6
3 suppliers
inquiry

2013-12-9
290 suppliers
$5.00/1g
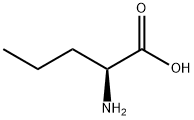
760-78-1
290 suppliers
$5.00/5g

2013-12-9
290 suppliers
$5.00/1g

1821-02-9
65 suppliers
$60.00/5mg

2013-12-9
290 suppliers
$5.00/1g