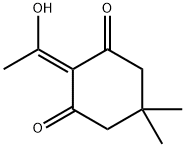
DDE-OH synthesis
- Product Name:DDE-OH
- CAS Number:94142-97-9
- Molecular formula:C10H14O3
- Molecular Weight:182.22
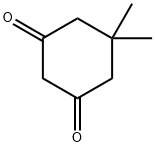
126-81-8
414 suppliers
$5.00/10g

75-36-5
561 suppliers
$17.92/100G

94142-97-9
104 suppliers
$22.00/1g
Yield: 77%
Reaction Conditions:
Stage #1:dimedone;acetyl chloride with pyridine in dichloromethane at 0 - 20; for 8 h;
Stage #2: with aluminum (III) chloride in dichloromethane at 20; for 7 h;
Steps:
synthesis of 2-acetyldimedone
The synthesis of 2-acetyldimedone (Dde-OH) was performed according to the literature with slight modifications.Dimedone(6.05 g, 43.2 mmol) and pyridine (3.83 mL, 47.5 mmol) were added to DCM (70 mL), and the mixture was cooled to 0 C. Acetyl chloride(3.73 mL, 10.1 mmol) was added, and stirring was continued at 0 C for 1 h. The reaction mixture was allowed to warm to room temperature, and stirred for 7 h. The mixture was diluted with DCM (50 mL), and the organic phase was washed with 1 M HCl(100 mL), sat. NaHCO3 (100 mL), and brine (100 mL). The solution was dried over Na2SO4, filtered, and concentrated. The resulting residue was dissolved in DCM (97 mL), and AlCl3 (12.21 g,91.6 mmol) was added. The reaction mixture was stirred at room temperature for 7 h, then poured into 6 M HCl (70 mL). The aqueous phase was extracted with DCM (100 mL 6) and AcOEt (100 mL 3). The combined organic layers were dried over Na2-SO4, filtered, and concentrated. The resulting residue was purified by silica gel column chromatography (10%-20% AcOEt/hexane) to give 2-acetyldimedone (6.05 g, 77%) as a pale yellow oil. Spectroscopicdata were identical to the published data.47 1H NMR(500 MHz, CDCl3): d 2.61 (s, 3H), 2.53 (s, 2H), 2.36 (s, 2H), 1.08(s, 6H), MS (m/z, EI): 182 [M]+.
References:
Amano, Yuichi;Umezawa, Naoki;Sato, Shin;Watanabe, Hisami;Umehara, Takashi;Higuchi, Tsunehiko [Bioorganic and Medicinal Chemistry,2017,vol. 25,# 3,p. 1227 - 1234]

108-24-7
5 suppliers
$14.00/250ML

126-81-8
414 suppliers
$5.00/10g

94142-97-9
104 suppliers
$22.00/1g

126-81-8
414 suppliers
$5.00/10g

64-19-7
1536 suppliers
$10.00/25ML

94142-97-9
104 suppliers
$22.00/1g