
Delpazolid synthesis
- Product Name:Delpazolid
- CAS Number:1219707-39-7
- Molecular formula:C14H17FN4O3
- Molecular Weight:308.31
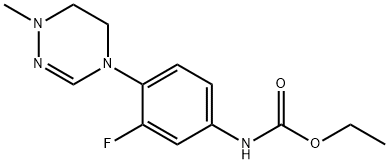
1334167-72-4
1 suppliers
inquiry
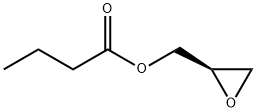
60456-26-0
311 suppliers
$14.14/1gm:

1219707-39-7
71 suppliers
$35.00/1mg
Yield: 67%
Reaction Conditions:
Stage #1:C13H17FN4O2 with methanol;lithium tert-butoxide in tetrahydrofuran;N,N-dimethyl-formamide at 0; for 0.5 h;
Stage #2:(R)-glycidyl butyrate in tetrahydrofuran;N,N-dimethyl-formamide at 20; for 10 h;
Steps:
1.1a
Synthesis of Compound 1aWhile the compound VI (30.5 g, 109 mmol) was put into a mixing solution of THF (300 mL) and DMF (150 mL) and was stirred, MeOH (8.8 mL, 218 mmol) and tBuOLi (26.1 g, 327 mmol) were slowly added thereto over 10 minutes at 0° C., followed by stirring for 20 minutes. (R)-glycidyl butyrate (31.4 mL, 218 mmol) was added to this solution and stirred for 10 hours at room temperature. Sat. NH4Cl (100 mL) was added to this solution and neutralized with 1N HCL, and the reactant was concentrated under reduced pressure. After the resultant was diluted with ethyl acetate (400 mL) and then was washed with distilled water (300 mL). The water layer was extracted again with dichloromethane (300 mL×4). After the organic layer was collected to be dried over Na2SO4, the resultant was filtered, followed by concentration under reduced pressure. Then, the filtrate was triturated with hexane and washed with ethylether. After this solid was heated again in isopropanol, a solid formed by cooling the resultant was filtered, thereby obtaining Compound 1a (22.5 g, 67%) as white solid.1H NMR (600 MHz, DMSO-d6) δ=7.59 (dd, J1=13.8 Hz, J2=2.4 Hz, 1H), 7.33-7.30 (m, 2H), 6.84 (s, 1H), 5.23 (t, J=5.4 Hz, 1H), 4.70 (m, 1H), 4.07 (t, J=9.0 Hz, 1H), 3.82 (m, 1H), 3.71 (t, J=4.8 Hz, 2H), 3.69-3.54 (m, 2H), 2.87 (t, J=4.8 Hz, 2H), 2.61 (s, 3H)LCMS: 309 (M+H+) for C14H17-FN4O3
References:
LEGOCHEM BIOSCIENCES, INC. US2013/5967, 2013, A1 Location in patent:Page/Page column 6-7

50-00-0
881 suppliers
$10.00/25g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1219707-39-7
71 suppliers
$35.00/1mg

369-34-6
411 suppliers
$5.00/5g

1219707-39-7
71 suppliers
$35.00/1mg
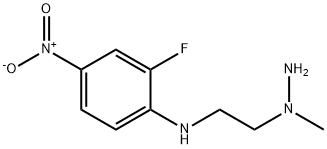
1334167-62-2
1 suppliers
inquiry

1219707-39-7
71 suppliers
$35.00/1mg
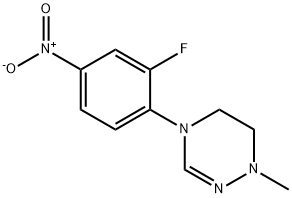
1334167-66-6
17 suppliers
inquiry

1219707-39-7
71 suppliers
$35.00/1mg