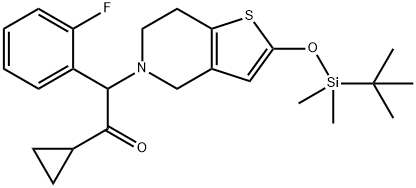
Desacetyl 2-O-tert-ButyldiMethylsilyl Prasugrel synthesis
- Product Name:Desacetyl 2-O-tert-ButyldiMethylsilyl Prasugrel
- CAS Number:952340-38-4
- Molecular formula:C24H32FNO2SSi
- Molecular Weight:445.67
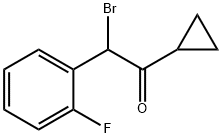
204205-33-4
238 suppliers
$30.00/5g
![5,6,7,7a-Tetrahydrothieno[3,2-c]pyridin-2(4H)-one](/CAS/GIF/109904-37-2.gif)
109904-37-2
42 suppliers
$75.00/100mg

18162-48-6
662 suppliers
$9.00/5g

952340-38-4
10 suppliers
$165.00/10mg
Yield:952340-38-4 90%
Reaction Conditions:
Stage #1: 5,6,7,7a-tetrahydrothieno[3,2-c]pyridine-2(4H)-one monohydrochloridewith triethylamine in dichloromethane at 10; for 0.25 h;
Stage #2: tert-butyldimethylsilyl chloride in dichloromethane at 10;
Stage #3: 2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanonewith triethylamine;sodium iodide in dichloromethane at 10 - 25;Temperature;
Steps:
4 The method for preparing 2-(tert-butyldimethylsilyloxy)-5-(α-cyclopropyl formyl-2-fluorobenzyl)-4,5,6,7-tetrahydro-thieno[3,2-c]pyridine (compound of Formula III)
Example 4
The method for preparing 2-(tert-butyldimethylsilyloxy)-5-(α-cyclopropyl formyl-2-fluorobenzyl)-4,5,6,7-tetrahydro-thieno[3,2-c]pyridine (compound of Formula III)
5,6,7,7a-Tetrahydro-4H-thieno[3,2-c]pyridin-2-one (2.0 g) was added to dichloromethane (10 mL) and stirred for 10 minutes at 10° C. Triethylamine (1.22 g) was then added and the mixture was stirred for 15 minutes to form a reaction mixture. Tert-butyldimethylsilyl chloride (1.81 g) dissolved in dichloromethane was added to the mixture at 10° C.
The mixture was stirred at 10° C. and the reaction was monitored by Thin Layer Chromatography (TLC) (methanol/ethyl acetate=3/7) until the reaction was completed.
Triethylamine (2.12 g), sodium iodide (0.02 g) and 2-bromo-2-(2-fluorophenyl)-1-cyclopropyl-ethanone (1.5 molar eq.) were added slowly to the reaction mixture at 10° C.
The reaction mixture was stirred at 25° C. and the reaction was monitored by Thin Layer Chromatography (TLC) (methanol/ethyl acetate=3/7).
After the reaction was completed, the organic phase was then washed with phosphate buffer (pH 6-7), and concentrated under reduced pressure at 30° C. to obtain 2-(tert-butyldimethylsilyloxy)-5-(α-cyclopropylformyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine as a crude product. The crude product (1 g) was recrystallized using acetone/water (1:1 v/v) (acetone volume=10 ml) at -5° C. for 3 hours for the crystallization method. Crystal precipitate was collected by filtration and dried under vacuum at 50° C. for 24 hours to obtain (tert-butyldimethylsilyloxy)-5-(α-cyclopropylformyl-2-fluorobenzyl)-4,5,6,7-tetrahydro-thieno[3,2-c]pyridine with the HPLC purity of 96%. The yield was 90%.
References:
US2014/155351,2014,A1 Location in patent:Paragraph 0083

178688-43-2
81 suppliers
inquiry
![4,5,6,7-Tetrahydrothieno[3,2-c]pyridin-2(3H)-one 4-methylbenzenesulfonate](/CAS2/GIF/178688-49-8.gif)
178688-49-8
42 suppliers
inquiry

18162-48-6
662 suppliers
$9.00/5g

952340-38-4
10 suppliers
$165.00/10mg

178688-43-2
81 suppliers
inquiry
![5,6,7,7a-Tetrahydrothieno[3,2-c]pyridin-2(4H)-one 4-methylbenzenesulfonate](/CAS/GIF/952340-39-5.gif)
952340-39-5
65 suppliers
$309.00/250mg

18162-48-6
662 suppliers
$9.00/5g

952340-38-4
10 suppliers
$165.00/10mg
![4,5,6,7-Tetrahydrothieno[3,2,c] pyridine hydrochloride](/CAS/GIF/28783-41-7.gif)
28783-41-7
337 suppliers
$5.00/5g

952340-38-4
10 suppliers
$165.00/10mg
451-82-1
344 suppliers
$6.00/5g

952340-38-4
10 suppliers
$165.00/10mg