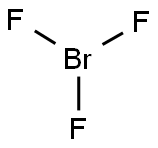Desflurane synthesis
- Product Name:Desflurane
- CAS Number:57041-67-5
- Molecular formula:C3H2F6O
- Molecular Weight:168.04
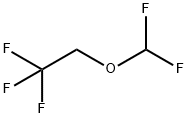
26675-46-7
234 suppliers
$19.00/1g
2837-89-0
2 suppliers
inquiry

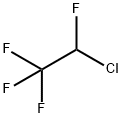
57041-67-5
55 suppliers
$195.00/10 g
Yield:57041-67-5 72.7%
Reaction Conditions:
with pyridine-hydrogen fluoride complex;hydrogen fluoride at 320;Inert atmosphere;Temperature;
Steps:
1; 2; 3; 4 Comparative Examples 1-4
Provided was a cylindrical reaction tube with an electric furnace as a gas-phase reactor (SUS 316L-made, diameter: 2.5 cm, length: 40 cm). The reaction tube was packed with 100 mL of the catalyst prepared in Preparation Example 1. The temperature of the reaction tube was raised to 180° C. while flowing nitrogen gas at a flow rate of about 10 mL/min through the reaction tube. Hydrogen fluoride was introduced into the reaction tube at a rate of about 0.1 g/min for 1 hour. Subsequently, 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether (isoflurane; 99.9 GC %) as a raw material was supplied at a rate of about 0.1 g/min (contact time: 25 seconds) into the reaction tube. The reaction became stable after the lapse of 1 hour from the initiation of the reaction. In this state, a gas flowing out of the reaction tube was blown into water to remove an acidic gas component. The thus-obtained product gas was analyzed by gas chromatography.
References:
Central Glass Company, Limited;HOSOI, Kenji;WATANABE, Mineo;IMURA, Hideaki;HIROTAKI, Kensuke;UESHIMA, Naoya US2019/345086, 2019, A1 Location in patent:Paragraph 0180-0183
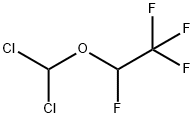
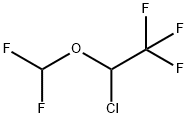
56885-29-1
0 suppliers
inquiry

57041-67-5
55 suppliers
$195.00/10 g

26675-46-7
234 suppliers
$19.00/1g

57041-67-5
55 suppliers
$195.00/10 g