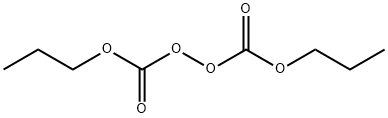
Di-n-propyl peroxydicarbonate synthesis
- Product Name:Di-n-propyl peroxydicarbonate
- CAS Number:16066-38-9
- Molecular formula:C8H14O6
- Molecular Weight:206.19

109-61-5
158 suppliers
$38.89/25g

16066-38-9
16 suppliers
inquiry
Yield: 63%
Reaction Conditions:
with sodium hydroxide;dihydrogen peroxide in isodecane;water at 21;
Steps:
For this preparation, a plate exchanger as represented in Figure 1, with a surface/volume ratio of 1800 m2/m3, comprising 3 inlet points 11 for the reactants, is used. Water cooled to 15°C, with a flow rate of 60 l/h, is used as utility for the cooling system (not represented in the figure). 5.5 l/h of a 7.8% NaOH sodium hydroxide solution are introduced continuously into the plate exchanger via point 11a, and 0.2 l/h of a 69.7% hydrogenperoxide H2O2 solution are introduced via point 11b. After the reaction which lasts approximately 6 seconds at 21°C, a mixture containing n-propylchloroformate and isodecane in proportions by mass of 88/12 is subsequently introduced continuously, via point 11c, at a flow rate of 1.2 l/h. The reaction is carried out at 21°C. A peroxide in solution in isodecane is obtained with a purity of 80% (corresponding to a purity of 96% of concentrated peroxide) and a yield of 63%, expressed relative to the chloroformate. The amount of n-propanol formed by hydrolysis in the aqueous phase is 1.45% relative to the amount of chloroformate.
References:
ARKEMA FRANCE EP1852418, 2007, A1 Location in patent:Page/Page column 6