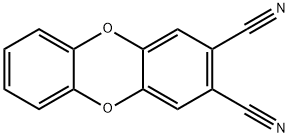
dibenzo-p-dioxin-2,3-dicarbonitrile synthesis
- Product Name:dibenzo-p-dioxin-2,3-dicarbonitrile
- CAS Number:309735-17-9
- Molecular formula:C14H6N2O2
- Molecular Weight:234.21
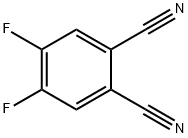
134450-56-9
128 suppliers
$40.00/500mg
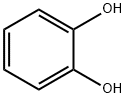
120-80-9
503 suppliers
$13.00/25g

309735-17-9
1 suppliers
inquiry
Yield:309735-17-9 43%
Reaction Conditions:
Stage #1: benzene-1,2-diolwith sodium hydride in N,N-dimethyl-formamide at 20; for 0.00833333 h;
Stage #2: 4,5-difluoro-1,2-dicyanobenzene in N,N-dimethyl-formamide at 20; for 0.166667 h;
Steps:
General procedure for synthesis of dibenzodioxins 7a-c
A solution of 5a (67 mg, 0.61 mmols) in DMF (10 mL) was treated NaH (60 %, 48mg, 1.2 mmols), allowed to stir for half a minute at room temperature followed by addition of phthalonitrile 6 (100 mg, 0.61 mmols). A deep blue coloration developed in the reaction flask and TLC monitoring in 10 minutes showed complete consumption of both starting materials 5a and 6 and appearance of a new spot more non-polar to both starting materials, which was very strongly UV-active. The reaction was quenched in cold water (100 mL), extracted into ethyl acetate (20 mL), organic layer repeatedly washed with brine solution (5 × 15 mL) and dried over sodium sulfate. Evaporation of the majority of ethyl acetate followed by re-crystallization in an ice-bath yielded 7a (single spot by TLC control, 30% Ethyl acetate/Petroleum ether) as buff-colored crystals. Yield (62 mg, 43%). 1H NMR (300 MHz, d6-DMSO): δ 7.82 (s, 2H), 7.05 (s, 4H); 13C NMR (150 MHz, d6-DMSO): δ 145.57, 140.06, 125.67, 121.99, 116.76, 115.27, 110.81; UV-vis (DMSO) λmax (log ε) 261 (4.53), 324 (3.48); HRMS (ESI+) calcd. for C14H6N2O2Na 257.0327, observed for C14H6N2O2Na 257.0293. Melting point: 311 °C.
References:
Banerjee, Subhadeep;Chattopadhyay, Anjan;Banerjee, Arnab;Haridas, Meera;Saini, Praveen;Das, Moitreyi;Majik, Mahesh S.;Maurya, Yogesh Kr. [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 4,p. 753 - 757] Location in patent:supporting information
139152-08-2
140 suppliers
$15.00/250mg

120-80-9
503 suppliers
$13.00/25g

309735-17-9
1 suppliers
inquiry
